Question: Please solve this problem and show all work: 13.35. The excess Gibbs energy for binary systems consisting of liquids not too dissimilar in chemical nature
Please solve this problem and show all work: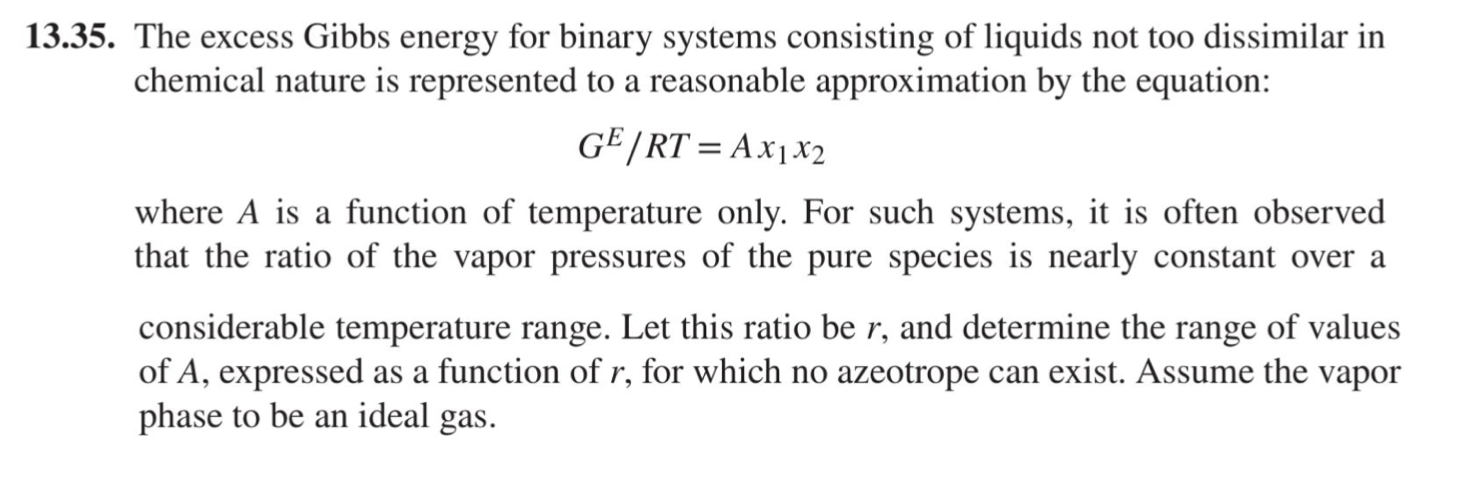
13.35. The excess Gibbs energy for binary systems consisting of liquids not too dissimilar in chemical nature is represented to a reasonable approximation by the equation: GE/RT = A x1x2 where A is a function of temperature only. For such systems, it is often observed that the ratio of the vapor pressures of the pure species is nearly constant over a considerable temperature range. Let this ratio be r, and determine the range of values of A, expressed as a function of r, for which no azeotrope can exist. Assume the vapor phase to be an ideal gas
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


