Question: Please solve this problem for a student to understand not for expert. Sometimes the answer is wrong. Please be careful. I will give you like
Please solve this problem for a student to understand not for expert. Sometimes the answer is wrong. Please be careful. I will give you like for you solution. Thanks!
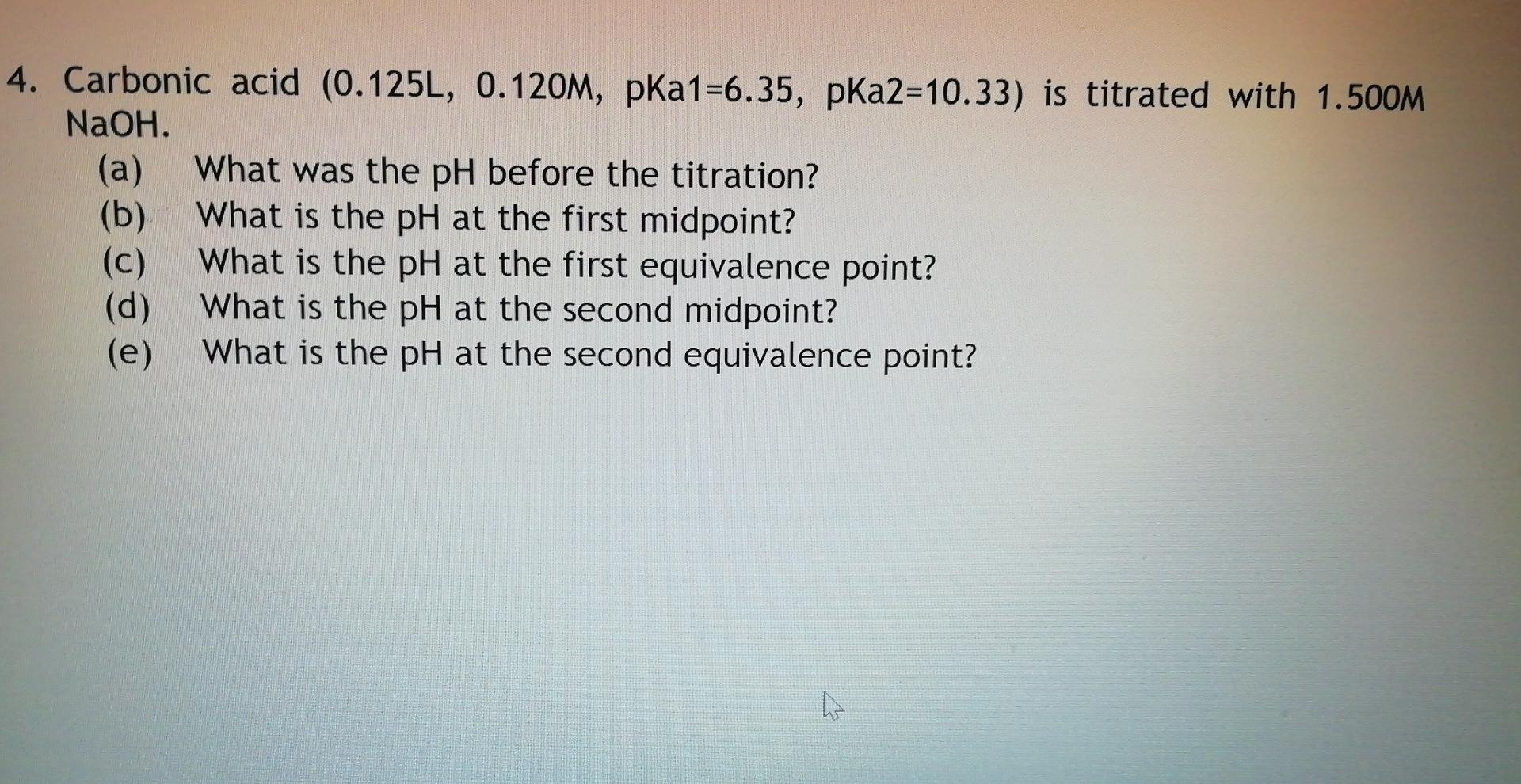
4. Carbonic acid (0.125L, 0.120M, pka1=6.35, pka2=10.33) is titrated with 1.500M NaOH. (a) What was the pH before the titration? (b) What is the pH at the first midpoint? (c) What is the pH at the first equivalence point? (d) What is the pH at the second midpoint? (e) What is the pH at the second equivalence point? W
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


