Question: please solve this problem in details. do not copy other tutor's solution please A+B+20 P12-21, OEQ (Old Exam Question). Also Hall of Fame Problem. The
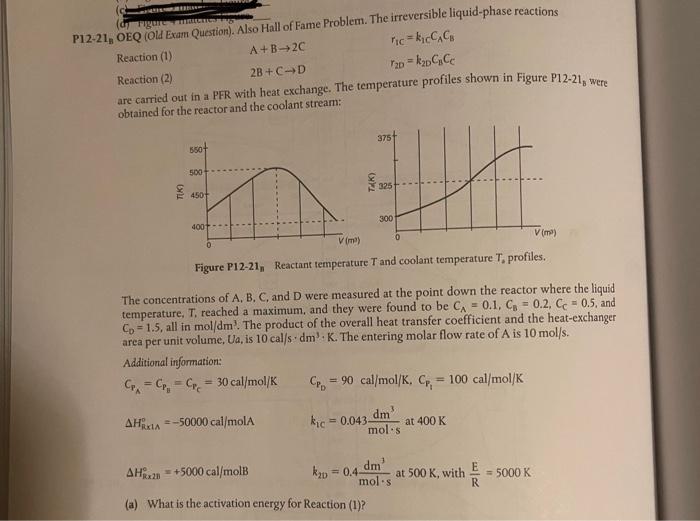
A+B+20 P12-21, OEQ (Old Exam Question). Also Hall of Fame Problem. The irreversible liquid-phase reactions Reaction (1) ricki CAC, Reaction (2) = k2o CCC are carried out in a PFR with heat exchange. The temperature profiles shown in Figure P12-21, were obtained for the reactor and the coolant stream: 2B+CD Tz 375 6501 500 N 325 4507 300 400 Vim) 0 Vime) Figure P12-21, Reactant temperature and coolant temperature T. profiles. The concentrations of A, B, C, and D were measured at the point down the reactor where the liquid temperature, T. reached a maximum, and they were found to be CA = 0.1, C = 0.2. Cc = 0.5, and Co - 1.5, all in mol/dm'. The product of the overall heat transfer coefficient and the heat-exchanger area per unit volume, Ua, is 10 calds. dm-K. The entering molar flow rate of A is 10 mol/s. Additional information: C = C, C = 30 cal/mol/K = 90 cal/mol/K, C, = 100 cal/mol/K AHA-50000 cal/mol kic=0.043 dm at 400 K mols AH... +5000 cal/mol k2u = 0.4- dm mol.s at 500 K, with = 5000 K R (a) What is the activation energy for Reaction (1)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


