Question: please solve this question and show all steps that's all things i can provide CE enters a combustion reactor with 25% excess air based on
please solve this question and show all steps
that's all things i can provide
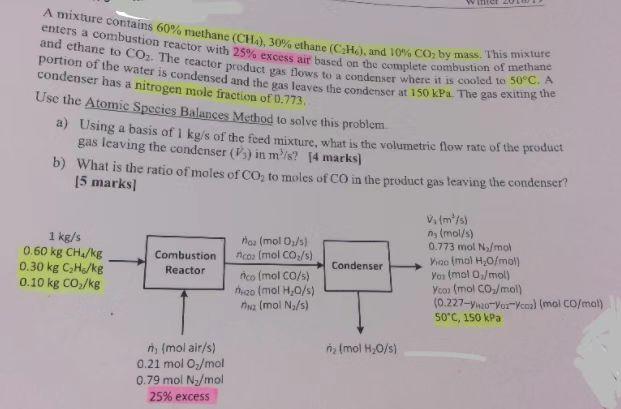
CE enters a combustion reactor with 25% excess air based on the complete combustion of methane A mixture contains 60% methane (CH4), 30% ethane (CH), and 10% CO by mass. This mixture and ethane to CO2. The reactor product pas bows to a condenser where it is cooled to 50C. A condenser has a nitrogen mole fraction of 0.773. the Use the Atomic Species Balances Method to solve this problem a) Using a basis of 1 kg/s of the feed mixture, what is the volumetric flow rate of the product gas leaving the condenser (s) in m/s? [4 marks] b) What is the ratio of moles of Coz to moles of CO in the product gas leaving the condenser? 15 marks 1 kg/s 0.60 kg CH /kg 0.30 kg CHE/kg 0.10 kg CO,/kg Combustion Reactor Condenser nos (mol 0/5) nco (mol Cos/s) nco (mol CO/5) ngoiol H2O/s) na (mol N./s) V. (m/s) na (mol/s) 0.773 mol N/mol Vico (mol H2O/mol) Yosmolo/mol) Yoon (mol CO./mol) (0.227-12-Yorkcoal (mol Co/mal) 50C, 150 kPa n (mol H20/s) (mol air/s) 0.21 mol 0/mol 0.79 mol Ny/mol 25% excess
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


