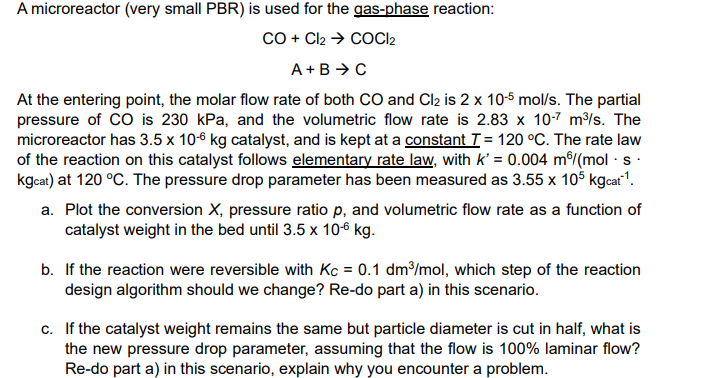
Question: Please solve using Polymath A microreactor ( very small PBR ) is used for the gas - phase reaction: C O + C l 2
Please solve using Polymath
A microreactor very small PBR is used for the gasphase reaction:
At the entering point, the molar flow rate of both and is The partial
pressure of is kPa, and the volumetric flow rate is The
microreactor has catalyst, and is kept at a constant The rate law
of the reaction on this catalyst follows elementary rate law, with
at The pressure drop parameter has been measured as
a Plot the conversion pressure ratio and volumetric flow rate as a function of
catalyst weight in the bed until
b If the reaction were reversible with which step of the reaction
design algorithm should we change? Redo part a in this scenario.
c If the catalyst weight remains the same but particle diameter is cut in half, what is
the new pressure drop parameter, assuming that the flow is laminar flow?
Redo part a in this scenario, explain why you encounter a problem.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


