Question: please solve with detailed solution and explanation 3. Bicine is a compound containing a tertiary amino group whose relevant pKa is 8.3. Given 1L of
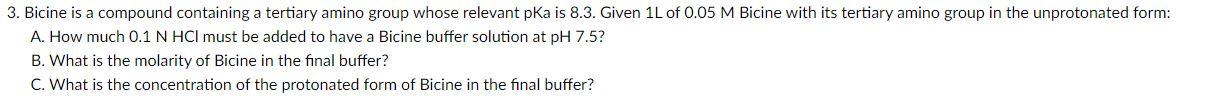 please solve with detailed solution and explanation
please solve with detailed solution and explanation
3. Bicine is a compound containing a tertiary amino group whose relevant pKa is 8.3. Given 1L of 0.05M Bicine with its tertiary amino group in the unprotonated form: A. How much 0.1NHCl must be added to have a Bicine buffer solution at pH7.5 ? B. What is the molarity of Bicine in the final buffer? C. What is the concentration of the protonated form of Bicine in the final buffer
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


