Question: please some do this step by step Total volume of reduced solution made (mL) Part B) Ion Exchange ( 0.5pt) Run #1: Volume of reduced
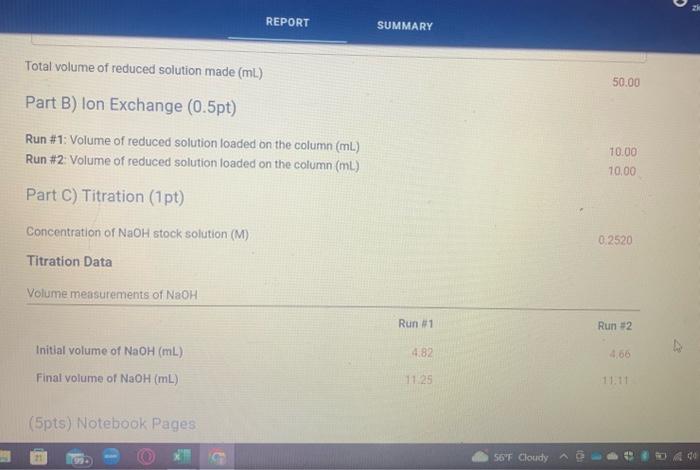
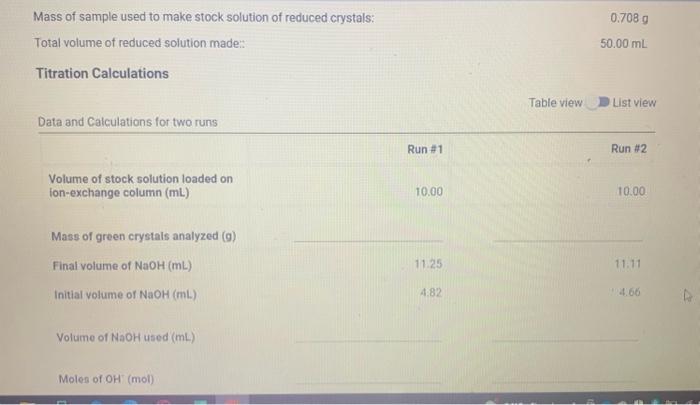
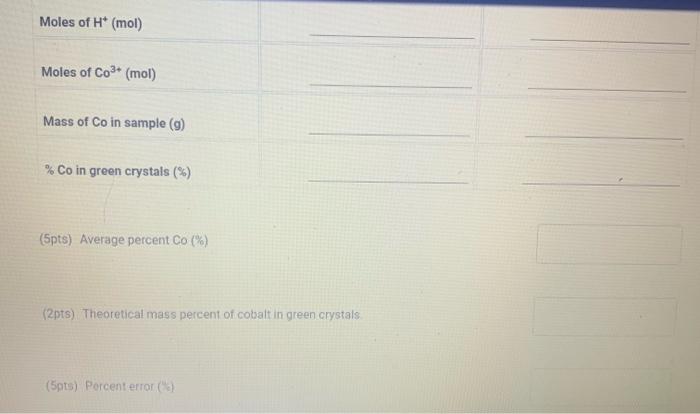
Total volume of reduced solution made (mL) Part B) Ion Exchange ( 0.5pt) Run \#1: Volume of reduced solution loaded on the column (mL) Run \#2: Volume of reduced solution loaded on the column (mL) Part C) Titration (1pt) Concentration of NaOH stock solution (M) Titration Data Volume measurements of NaOH Initial volume of NaOH(mL) Final volume of NaOH(mL) (5pts) Notebook Pages Mass of sample used to make stock solution of reduced crystals: Total volume of reduced solution made: 50.00mL Titration Calculations Table view List view Moles of H+(mol) Moles of Co3+(mol) Mass of Co in sample (g) % Co in green crystals ( % ) (5pts) Average percent Co (\%) (2pts) Theoretical mass percent of cobalt in green crystals (5pts) Porcent error ( ) )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


