Question: Please type the answer/ write nice so i can understand and answer all the questions. thank you i need you to draw a picture/ diagrom
Please type the answer/ write nice so i can understand and answer all the questions. thank you
i need you to draw a picture/ diagrom of theh procedure and the set up.
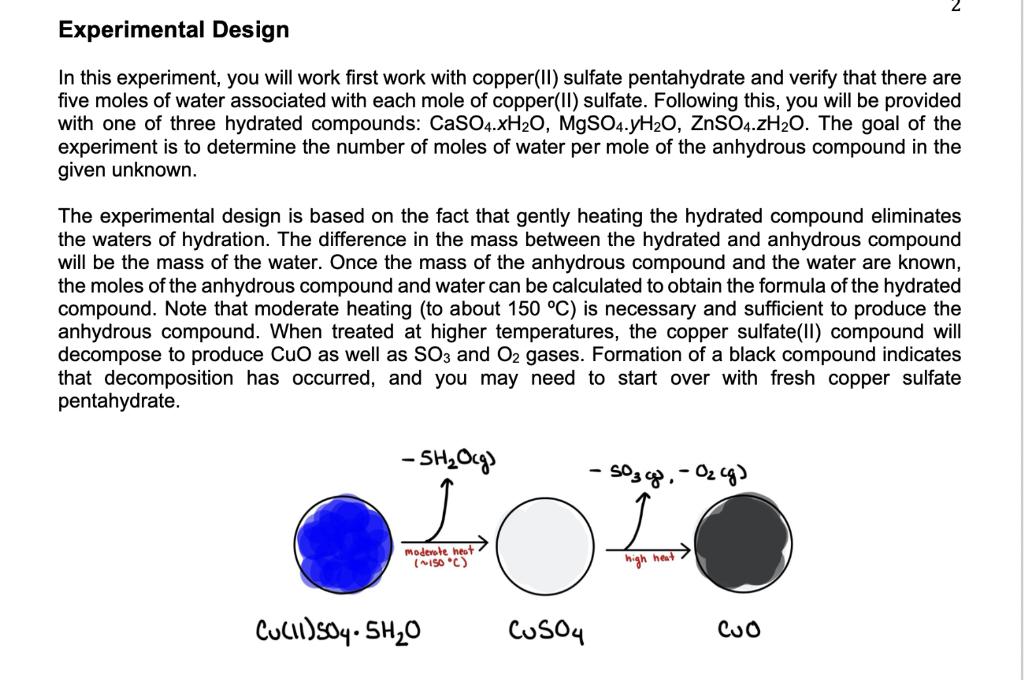


Experimental Design In this experiment, you will work first work with copper(II) sulfate pentahydrate and verify that there are five moles of water associated with each mole of copper(II) sulfate. Following this, you will be provided with one of three hydrated compounds: CaSO4.XH20, MgSO4.7H20, ZnSO4.7H20. The goal of the experiment is to determine the number of moles of water per mole of the anhydrous compound in the given unknown. The experimental design is based on the fact that gently heating the hydrated compound eliminates the waters of hydration. The difference in the mass between the hydrated and anhydrous compound will be the mass of the water. Once the mass of the anhydrous compound and the water are known, the moles of the anhydrous compound and water can be calculated to obtain the formula of the hydrated compound. Note that moderate heating (to about 150 C) is necessary and sufficient to produce the anhydrous compound. When treated at higher temperatures, the copper sulfate(II) compound will decompose to produce CuO as well as SO3 and O2 gases. Formation of a black compound indicates that decomposition has occurred, and you may need to start over with fresh copper sulfate pentahydrate. - 5H2Ocg) -S03cg. - Oz cg) 1 Moderate heat (150C) high heut Culli) s04.5H20 CuSO4 CUO Reagents and Supplies Chemicals CuSO4.5H20 and one of CaSO4.XH2O, MgSO4.yH20, ZnSO4.7H20 (all solids) Safety Copper sulfate pentahyrate is toxic when swallowed or inhaled, and causes skin irritation. It is also very toxic to aquatic life with long lasting effects. Wear appropriate PPE when handling copper sulfate pentahydrate and dispose of your materials in the chemical waste. Do not ever pour chemicals down the sink. See posted Material Safety Data Sheets for more information. Equipment Micro-crucibles Crucible tongs Hot plates Procedure PART 1: ANALYSIS OF COPPER (II) SULFATE PENTAHYDRATE 1. Heat an empty crucible and bring it to a constant mass (+0.0010 g). In order to do this, record the mass of the empty crucible. Then heat the crucible for 3-4 minutes. Note that the surface of the hot plate reaches ~500 C when it is turned on all the way. Go low and slow and keep the temperature of the hot plate lower than 250 C. 2. Cool the crucible on the lab bench and record its dry mass. Handle the microcrucible with crucible tongs (it is very hot!). For winter 2022, you may heat the microcrucible only once to ensure excess water has been removed. Make sure that you record the dry mass of the microcrucible in your lab notebook, 3. Add approximately 1.00 grams of copper(II) sulfate pentahydrate to the micro-crucible. Measure the mass of the micro-crucible with the solid. Record this value in your lab notebook. 4. Heat the micro-crucible over a hot plate until the blue color is gone. 5. Cool the micro-crucible to room temperature and measure the mass of the micro-crucible with the anhydrous compound. Record this value in your lab notebook. 6. Discard the contents of the micro-crucible in the appropriate waste disposal container. Double check with your instructor if you are unsure which waste container to use
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


