Question: Please type work, I will give you thumbs up, thank you. 3. a) What is the stoichiometric or equivalence point in an acid-base titration? b)
Please type work, I will give you thumbs up, thank you.
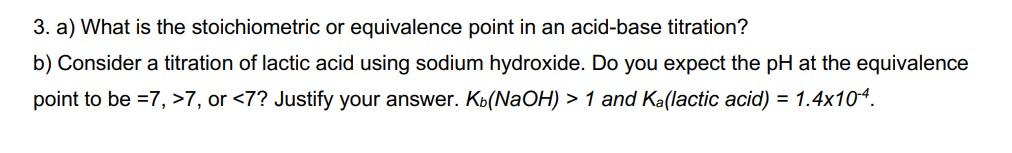
3. a) What is the stoichiometric or equivalence point in an acid-base titration? b) Consider a titration of lactic acid using sodium hydroxide. Do you expect the pH at the equivalence point to be =7,>7, or 1 and Ka (lactic acid) =1.4104
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


