Question: PLEASE TYPING THE ANSWER, NOT WRITING al) Explain why Phase Diagrams are so important in the process industry; and a2) Describe in detail what happens
 PLEASE TYPING THE ANSWER, NOT WRITING
PLEASE TYPING THE ANSWER, NOT WRITING
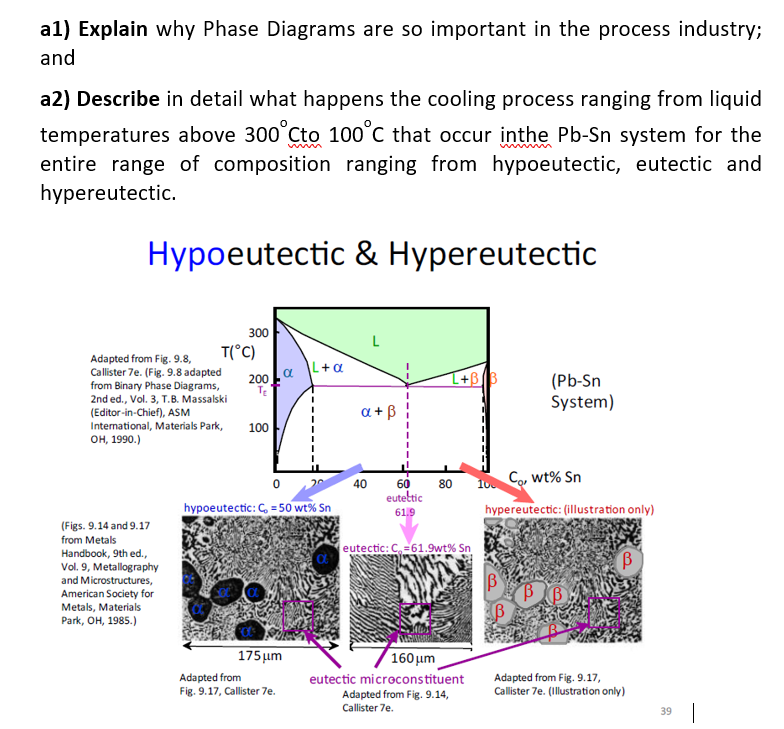
al) Explain why Phase Diagrams are so important in the process industry; and a2) Describe in detail what happens the cooling process ranging from liquid temperatures above 300to 100c that occur inthe Pb-Sn system for the entire range of composition ranging from hypoeutectic, eutectic and hypereutectic. Hypoeutectic & Hypereutectic L+a 300 Adapted from Fig. 9.8. T(C) Callister 7e. (Fig. 9.8 adapted 200 from Binary Phase Diagrams, 2nd ed., Vol. 3, T.B. Massalski (Editor-in-Chief), ASM International, Materials Park, 100 OH, 1990.) L+B) (Pb-Sn System) 1 a + B 1 40 80 10 Co, wt% Sn 0 hypoeutectic: C = 50 wt% Sn 60 euteltic 61.9 hypereutectic: (illustration only) eutectic: C61.9wt% Sn B (Figs. 9.14 and 9.17 from Metals Handbook, 9th ed. Vol. 9, Metallography and Microstructures, American Society for Metals, Materials Park, OH, 1985.) 175 um Adapted from Fig. 9.17, Callister 7e. 160 um eutectic microconstituent Adapted from Fig. 9.14, Callister 7e. Adapted from Fig. 9.17, Callister 7e. (Illustration only) 39 |
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


