Question: PLEASE USE EVERYTHING GIVEN TO ANSWER PROBLEMS PROPERLY THANK YOU PLEASE USE EVERYTHING GIVEN TO ANSWER PROBLEMS PROPERLY THANK YOU Basic Equations: SIM At T=T,
PLEASE USE EVERYTHING GIVEN TO ANSWER PROBLEMS PROPERLY THANK YOU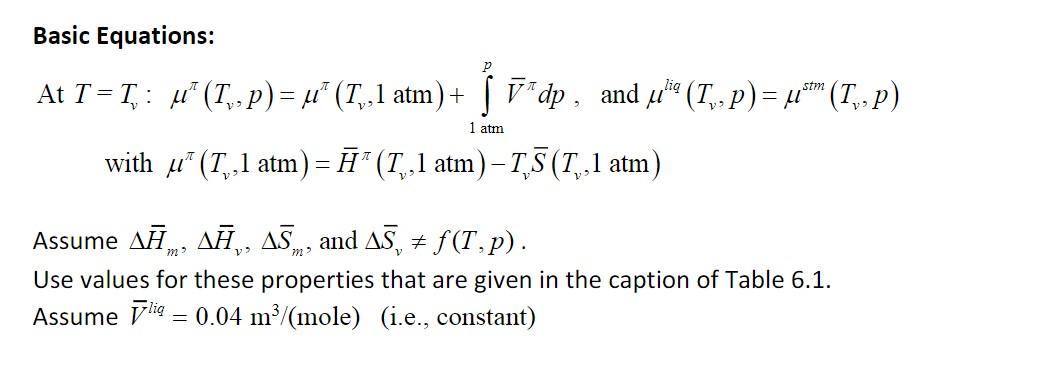
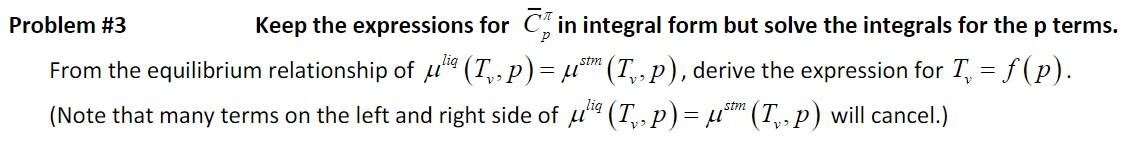
 PLEASE USE EVERYTHING GIVEN TO ANSWER PROBLEMS PROPERLY THANK YOU
PLEASE USE EVERYTHING GIVEN TO ANSWER PROBLEMS PROPERLY THANK YOU
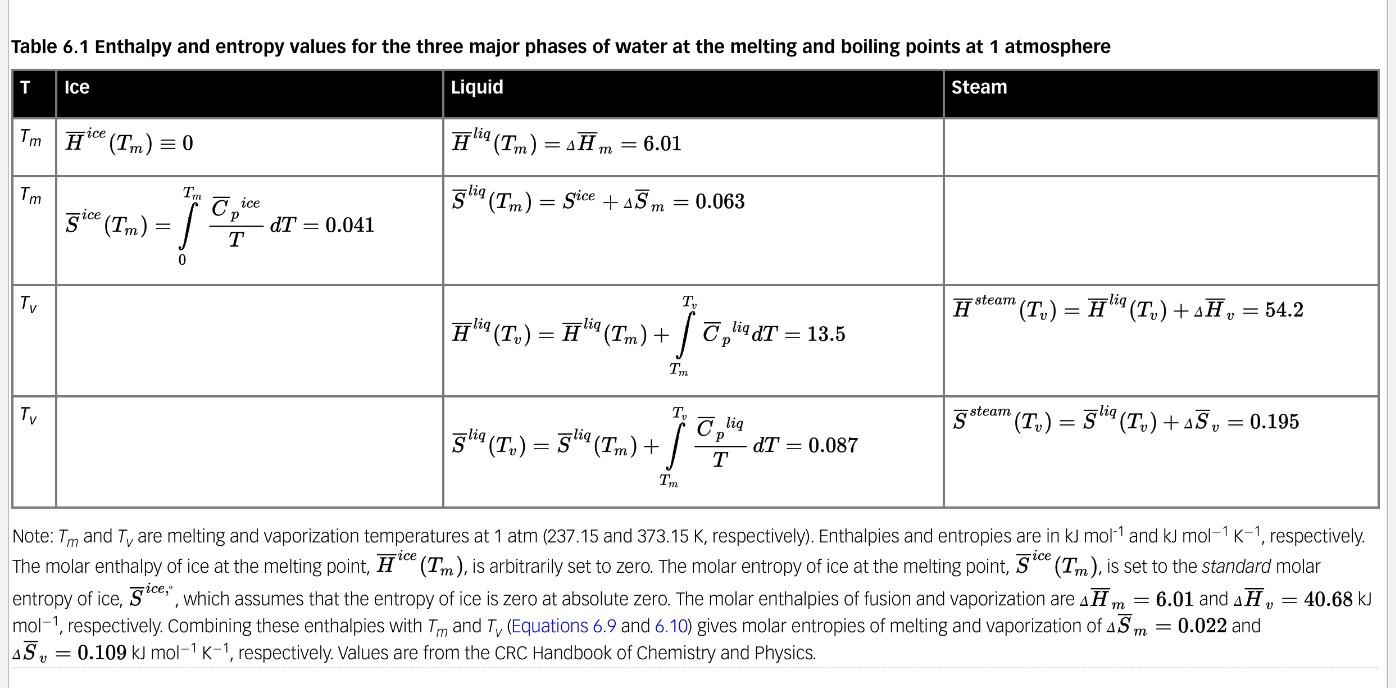
Basic Equations: SIM At T=T, : u" (Tv, p) = u (Tv,1 atm) + vdp, and fulie (7,- p) = 4*m (P) 1 atm with u (T, ,1 atm) = " (T,1 atm) T, 5 (T1,1 atm) Assume Am, A,, 45., and A5, 7, f(T,p). Use values for these properties that are given in the caption of Table 6.1. Assume lig 0.04 m3/(mole) (i.e., constant) Problem #3 Keep the expressions for Cj in integral form but solve the integrals for the p terms. From the equilibrium relationship of helico (Tv,p) = * (Tv-p), derive the expression for T, = f(p). (Note that many terms on the left and right side of ulio (T., p) = * (Tv,p) will cancel.) stm = stm Table 6.1 Enthalpy and entropy values for the three major phases of water at the melting and boiling points at 1 atmosphere T Steam Ice Liquid Tm Hice (Tm) = 0 #liq(Tm) = xH m = 6.01 Tm TR ice glig (Tm) Cp = gice +AS m = 0.063 3i (Tn) = ! ice = T = 0.041 T 0 Tv T H steam (T,) = 7"! (T,) +-7, = 54.2 lig Hliq (T,) = Aliq(Tm) + jo C, lidt 13.5 TO Tv T lig S steam "(T,) = gliq(T,) +15, = 0.195 gliq (T,) = liq '(Tm) + +e;" 0T = 0.087 T TM Note: Tm and T are melting and vaporization temperatures at 1 atm (237.15 and 373.15 K, respectively). Enthalpies and entropies are in kJ mol and kJ mol-1 K-1, respectively. The molar enthalpy of ice at the melting point, Hice (Tm), is arbitrarily set to zero. The molar entropy of ice at the melting point, gice (Tm), is set to the standard molar entropy of ice, gice,", which assumes that the entropy of ice is zero at absolute zero. The molar enthalpies of fusion and vaporization are AH, = 6.01 and AH, = 40.68 kJ mol-1, respectively. Combining these enthalpies with Tm and TV (Equations 6.9 and 6.10) gives molar entropies of melting and vaporization of Sm = 0.022 and AS, = 0.109 kJ mol-1 K-1, respectively. Values are from the CRC Handbook of Chemistry and Physics. m
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


