Question: Please use excel A surface water sample at 18C has an alkalinity of 140mg/L as CaCO3, and a C of 6.35102M. Assuming the only species
 Please use excel
Please use excel
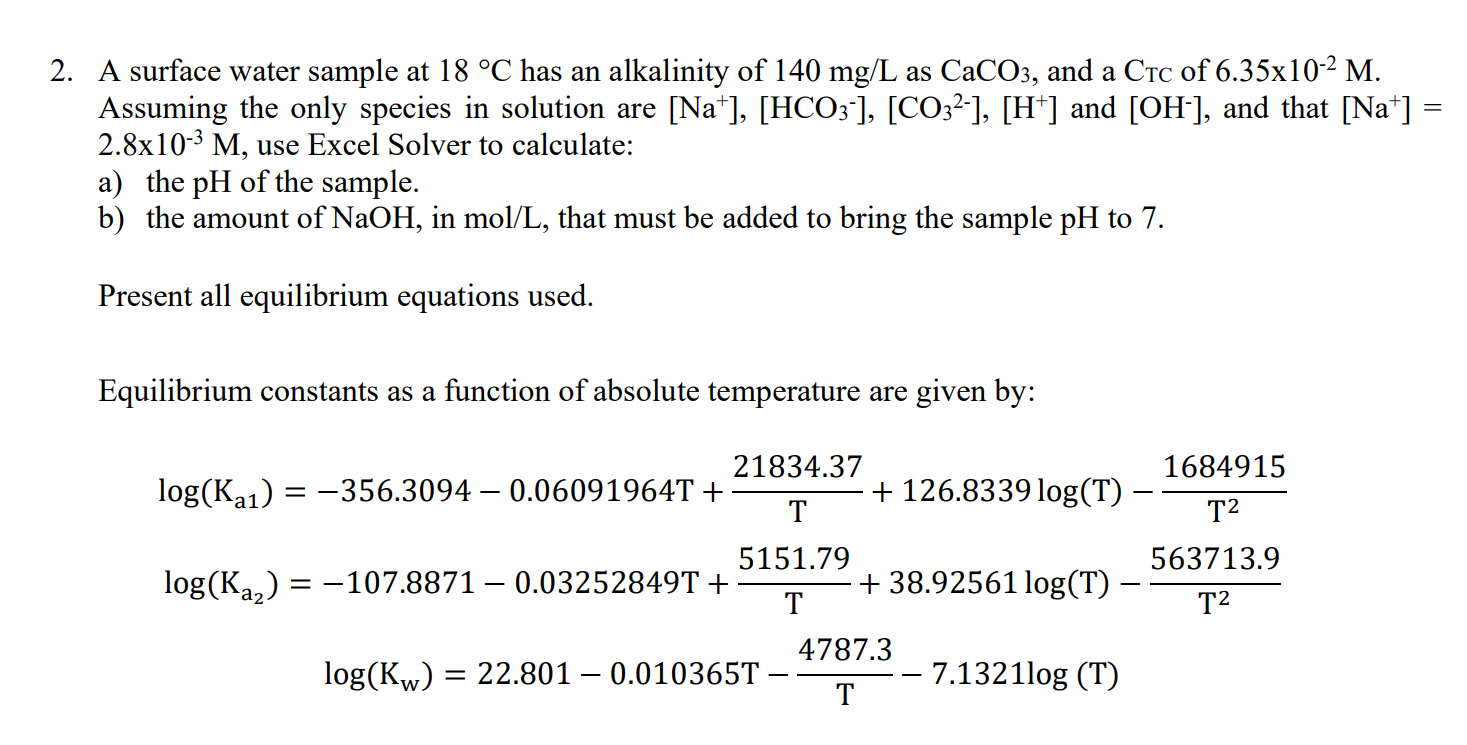
A surface water sample at 18C has an alkalinity of 140mg/L as CaCO3, and a C of 6.35102M. Assuming the only species in solution are [Na+],[HCO3],[CO32],[H+]and [OH], and that [Na+]= 2.8103M, use Excel Solver to calculate: a) the pH of the sample. b) the amount of NaOH, in mol/L, that must be added to bring the sample pH to 7 . Present all equilibrium equations used. Equilibrium constants as a function of absolute temperature are given by: log(Ka1)=log(Ka2)=356.30940.06091964T+T21834.37+126.8339log(T)T21684915107.88710.03252849T+T5151.79+38.92561log(T)T2563713.9log(Kw)=22.8010.010365TT4787.37.1321log(T)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


