Question: PLEASE USE POLYMATH AND SHOW CODES !!! Problem 3: Elements of Chemical Reaction Engineering 6th Ed. P8-9 (a and b) P8-98 OEQ (Old Exam Question).
PLEASE USE POLYMATH AND SHOW CODES !!!
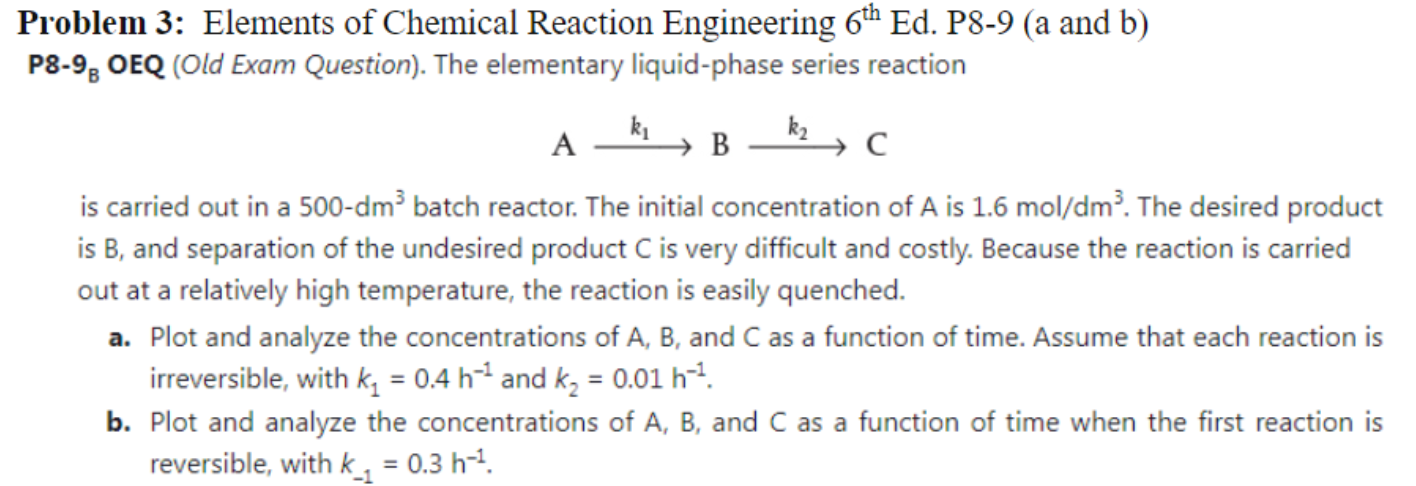
Problem 3: Elements of Chemical Reaction Engineering 6th Ed. P8-9 (a and b) P8-98 OEQ (Old Exam Question). The elementary liquid-phase series reaction A B is carried out in a 500-dm batch reactor. The initial concentration of A is 1.6 mol/dm. The desired product is B, and separation of the undesired product C is very difficult and costly. Because the reaction is carried out at a relatively high temperature, the reaction is easily quenched. a. Plot and analyze the concentrations of A, B, and C as a function of time. Assume that each reaction is irreversible, with kq = 0.4 h-4 and kz = 0.01 h-7. b. Plot and analyze the concentrations of A, B, and C as a function of time when the first reaction is reversible, with k_1 = 0.3 h-4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


