Question: please use the given number provided in the picture. Table 1: Standard solution for FeNCS2+ ion Beer's plot Solution SI (blank) S2 S3 54 S5
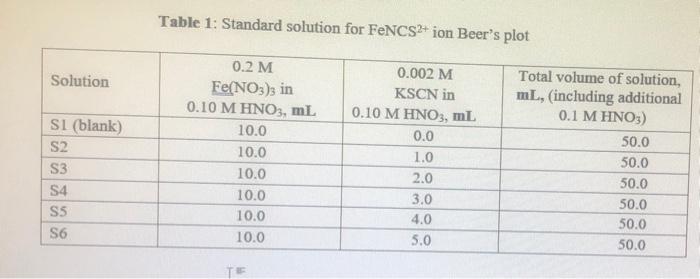
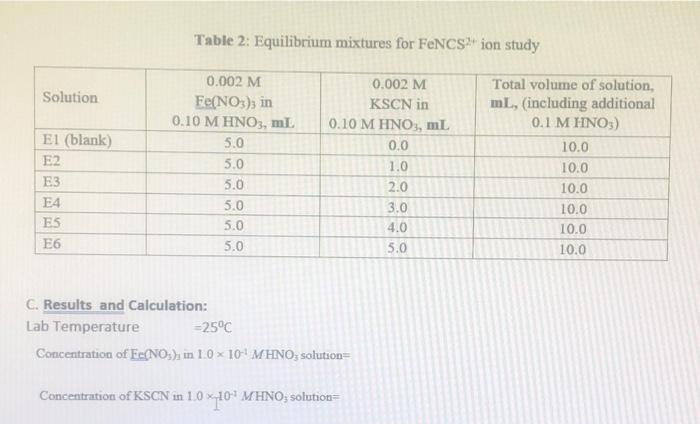
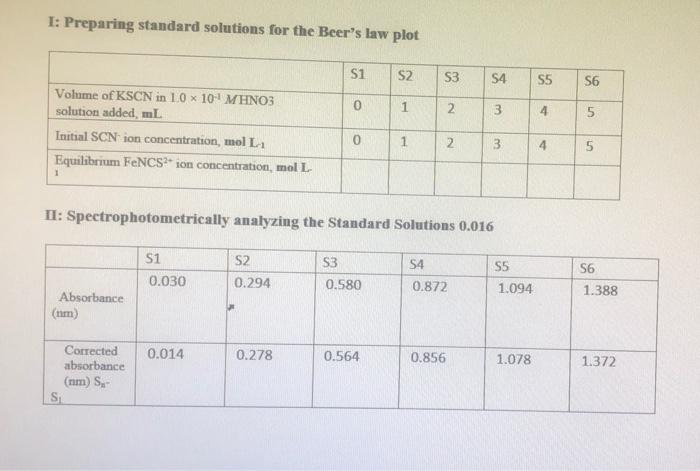
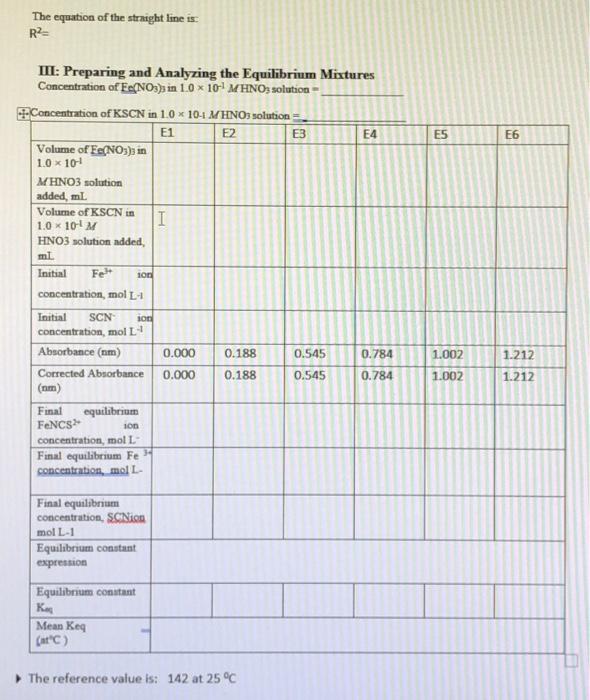
Table 1: Standard solution for FeNCS2+ ion Beer's plot Solution SI (blank) S2 S3 54 S5 S6 0.2 M Fe(NO3)3 in 0.10 M HNO3, ml 10.0 10.0 10.0 10.0 10.0 10.0 0.002 M KSCN in 0.10 M HNO3, ml 0.0 1.0 2.0 3.0 4.0 5.0 Total volume of solution, mL, (including additional 0.1 M HNO3) 50.0 50.0 50.0 50.0 50.0 50.0 TE Table 2: Equilibrium mixtures for FeNCS ion study Solution 0.002 M Fe(NO3)3 in 0.10 M HNO3, mL 5.0 5.0 El (blank) E2 E3 E4 ES E6 0.002 M KSCN in 0.10 M HNO3, ml 0.0 1.0 2.0 3.0 4.0 5.0 Total volume of solution, mL, (including additional 0.1 M HNO3) 10.0 10.0 10.0 10.0 10.0 10.0 5.0 5.0 5.0 . 5.0 C. Results and Calculation: Lab Temperature =25C Concentration of Fe(NOx), in 10 x 10-4 MHNO solution= Concentration of KSCN in 1.0 x 10' MHNO; solution= I: Preparing standard solutions for the Beer's law plot S1 S2 53 S5 S6 54 3 0 1 2 3 4 5 Volume of KSCN in 10 x 10-4 M HNO3 solution added, mL Initial SCN ion concentration, mol L Equilibrium FeNCS ion concentration, mol L 0 1 2 3 4 5 1 II: Spectrophotometrically analyzing the Standard Solutions 0.016 S1 0.030 S2 0.294 S3 0.580 S4 0.872 S5 1.094 S6 1.388 Absorbance 0.014 0.278 0.564 0.856 1.078 1.372 Corrected absorbance (nm) S SI The equation of the straight line is R2- X 4 E5 E6 TII: Preparing and Analyzing the Equilibrium Mixtures Concentration of Fe(NO3)3 in 1.0 * 10 MHNO3 solution- Concentration of KSCN in 10 x 10-1 MHNOs solution E1 E2 E3 Volume of Fe(NO3)3 in 1.0 * 101 MHNO3 solution added, ml Volume of KSCN in I 1.0 * 101 M HNO3 solution added ml Initial 10 concentration, mol Total SCN concentration, molt Absorbance (nm) 0.000 0.188 0.545 0.784 Corrected Absorbance 0.000 0.188 0.545 0.784 (nm) Final equilibrium FENCS son concentration, moll Final equilibrium Fe concentration, moll Felt 1.002 1.212 1.002 1.212 Final equilibrium concentration, SC in mol L-1 Equilibrium constant expression Equilibrium constant Ka Mean Kec (at) The reference value is: 142 at 25C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


