Question: Please use the information in part A) to answer part B) and C) Calculate the standard enthalpy change for the reaction 2A+B2C+2D where the heats
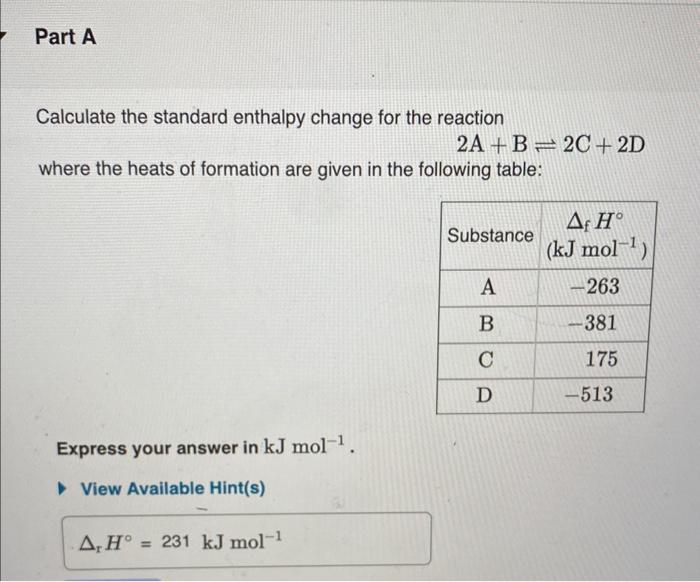
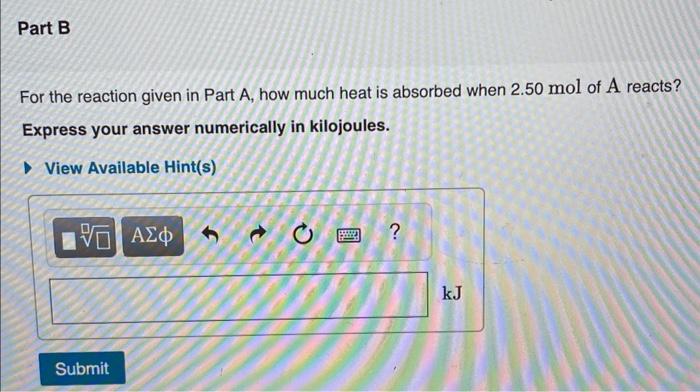
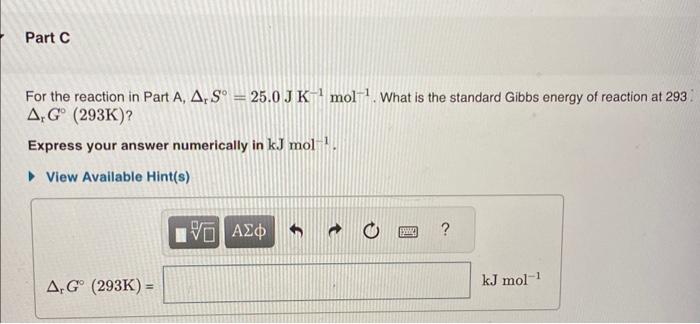
Calculate the standard enthalpy change for the reaction 2A+B2C+2D where the heats of formation are given in the following table: For the reaction given in Part A, how much heat is absorbed when 2.50mol of A reacts? Express your answer numerically in kilojoules. For the reaction in Part A, rS=25.0JK1mol1. What is the standard Gibbs energy of reaction at 293 . rG(293K) ? Express your answer numerically in kJmol1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


