Question: Please use the numbers that are highlighted in yellow & answer all questions. Thank you! A continuous, steady-state distillation column with a total condenser and
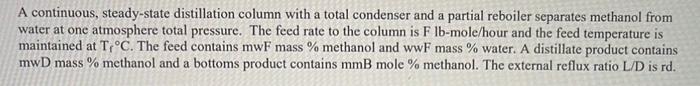
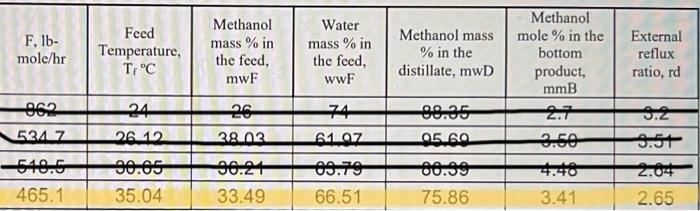
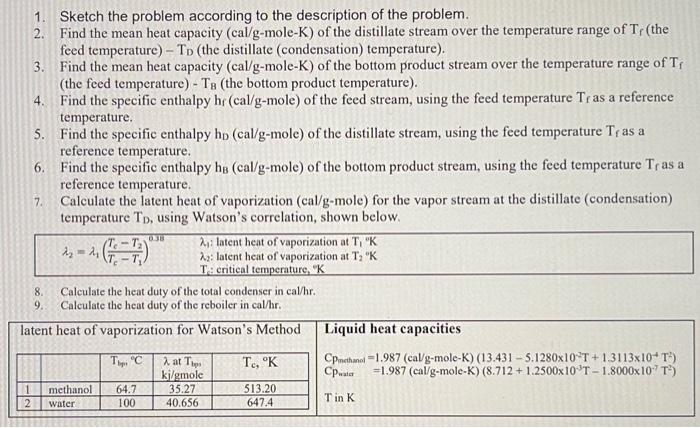
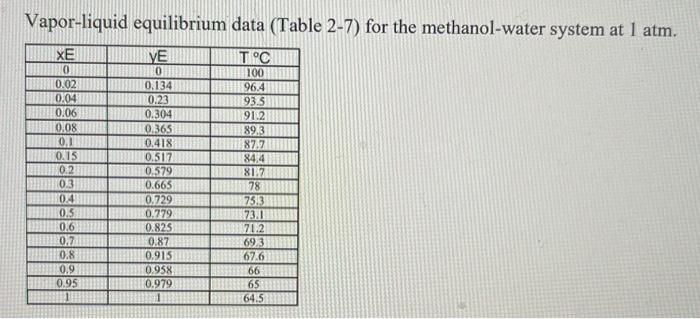
A continuous, steady-state distillation column with a total condenser and a partial reboiler separates methanol from water at one atmosphere total pressure. The feed rate to the column is F lb-mole/hour and the feed temperature is maintained at T;C. The feed contains mwF mass % methanol and wwf mass % water. A distillate product contains mwD mass % methanol and a bottoms product contains mmB mole % methanol. The external reflux ratio L/D is rd. F.lb- mole/hr Feed Temperature, T,"C Methanol mass % in the feed, mwF Water mass % in the feed, wwF Methanol mass % in the distillate, mwD Methanol mole % in the bottom product, mmB External reflux ratio, rd 862 74 24 26. 12 26 29.02 5247 6197 05.00 9.50 9.2 9.5+ 2.04 2.65 540.6 465.1 90.05 35.04 96.2+ 33.49 09.70 66.51 . 75.86 3.41 1. Sketch the problem according to the description of the problem. 2. Find the mean heat capacity (cal/g-mole-K) of the distillate stream over the temperature range of T (the feed temperature) - To the distillate (condensation) temperature) 3. Find the mean heat capacity (cal/g-mole-K) of the bottom product stream over the temperature range of T, (the feed temperature) - T. (the bottom product temperature). 4. Find the specific enthalpy hr(cal/g-mole) of the feed stream, using the feed temperature Tras a reference temperature. 5. Find the specific enthalpy hp (cal/g-mole) of the distillate stream, using the feed temperature Tras a reference temperature. 6. Find the specific enthalpy hs (cal/g-mole) of the bottom product stream, using the feed temperature Tras a reference temperature. 7. Calculate the latent heat of vaporization (cal/g-mole) for the vapor stream at the distillate (condensation) temperature To, using Watson's correlation, shown below. -16:= 2, latent heat of vaporization at T, "K 22; latent heat of vaporization at T; "K T: critical temperature, "K Calculate the heat duty of the total condenser in cal/hr. 9. Calculate the heat duty of the reboiler in cal/hr. latent heat of vaporization for Watson's Method Liquid heat capacities TC TC, "K CPnathanol -1.987 (cal/g-mole-K) (13.431 - 5.1280x10T +1.3113x10* T) ki/gmole =1.987 (cal/g-mole-K) (8.712 + 1.2500x10T - 1.8000x10-T) 1 methanol 64.7 35.27 513.20 100 40.656 647.4 Tin K 2 8. 2 at The 2 water Vapor-liquid equilibrium data (Table 2-7) for the methanol-water system at 1 atm. XE 0 0.02 0.04 0.06 0.08 0.1 0.15 0.2 0.3 0.4 0.5 0.6 M 0.7L 0.8 0.9 0.95 13 VE TIC 0 100 0.134 96.4 0.23 93.5 0.304 91.2 0.365 W 89.3 0.418 87.7 0.517 13 84,4 0.579 81.7 0.665 78 0.729 73.3 0.779 73.1 0.825133371.2 0.87 69.3 0.915 67.6 0.958 66 0.979 65 3 64.5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


