Question: Please use the values in the resources listed below instead of the textbook values. (a) An important source of copper is from the copper ore,
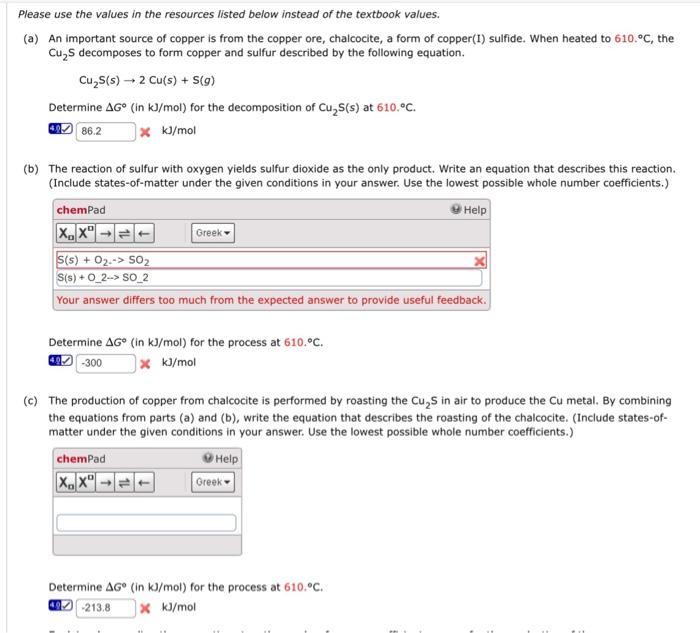
Please use the values in the resources listed below instead of the textbook values. (a) An important source of copper is from the copper ore, chalcocite, a form of copper(I) sulfide. When heated to 610.C, the Cu2S decomposes to form copper and sulfur described by the following equation. Cu2S(s)2Cu(s)+S(g) Determine G (in kJ/mol ) for the decomposition of Cu2S(s) at 610.C. * kJ/mol (b) The reaction of sulfur with oxygen yields sulfur dioxide as the only product. Write an equation that describes this reaction. (Include states-of-matter under the given conditions in your answer. Use the lowest possible whole number coefficients.) Determine G (in kJ/mol) for the process at 610.C. * kJ/mol (c) The production of copper from chalcocite is performed by roasting the Cu2S in air to produce the Cu metal. By combining the equations from parts (a) and (b), write the equation that describes the roasting of the chalcocite. (Include states-ofmatter under the given conditions in your answer. Use the lowest possible whole number coefficients.) Determine G (in kJ/mol ) for the process at 610.C. kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


