Question: PLEASE USE VALUES FROM PROBLEM. do not copy other solutions please!!!. Carbon dioxide (CO2) fills a closed, rigid tank fitted with a paddle wheel, initially
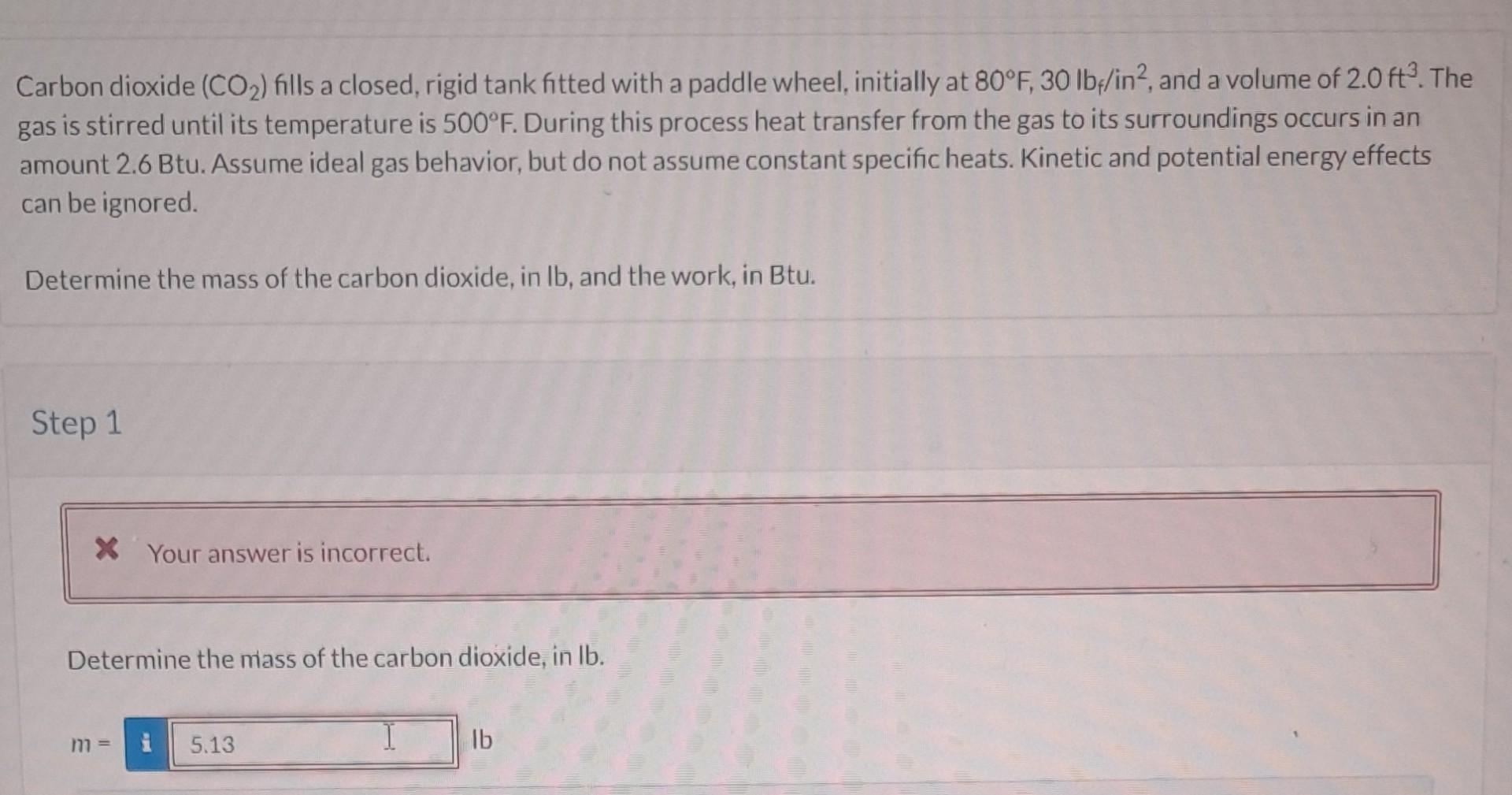
PLEASE USE VALUES FROM PROBLEM. do not copy other solutions please!!!.
Carbon dioxide (CO2) fills a closed, rigid tank fitted with a paddle wheel, initially at 80F,30lbf/in2, and a volume of 2.0ft3. The gas is stirred until its temperature is 500F. During this process heat transfer from the gas to its surroundings occurs in an amount 2.6 Btu. Assume ideal gas behavior, but do not assume constant specific heats. Kinetic and potential energy effects can be ignored. Determine the mass of the carbon dioxide, in Ib, and the work, in Btu. Step 1 Determine the mass of the carbon dioxide, in lb
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


