Question: please work stoichiometrically. not sure what i am doing wrong. Janda Aw Your Chan ..MY 35 0.400 M., 20.30H. MACH SO, NO C:0,20 HO Dece|
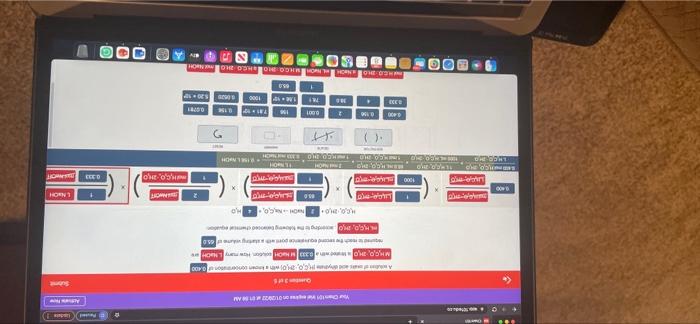
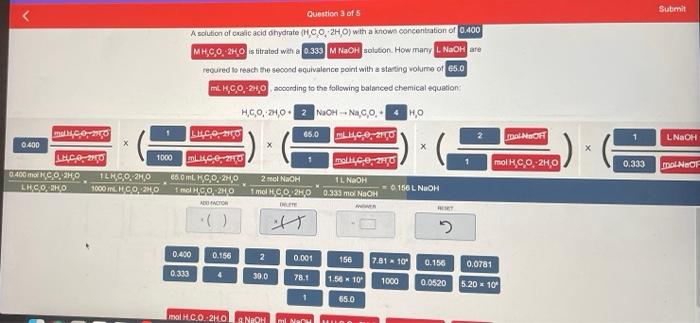
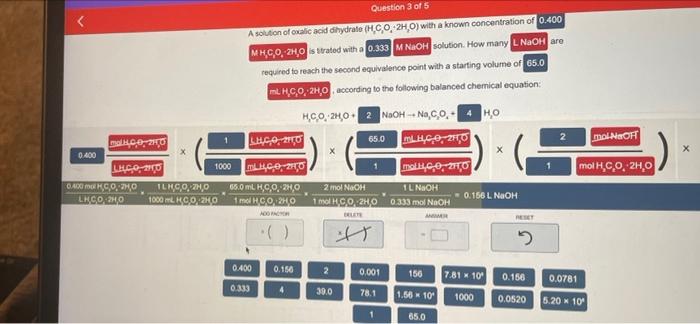
Janda Aw Your Chan ..MY 35 0.400 M., 20.30H. MACH SO, NO C:0,20 HO Dece| MWC LO 1000 LIGGO moko, 20 0.2 RICO, CON TE Olar RECORT 1000 so 6.2006 156 16 TAS 1 2.30 741 1000 6000 5.20 65.0 REDONDONGOLARCOOL Submit Question 3 of 5 A solution of Onic acid hydrate (CO 2HO) with a known concentration of 0.400 MHCO, HO is titrated wana 0.333 MNAOH solution. How many. NaOH are recured to reach the second equivalence point with a starting volume of 65.0 m. H.C.0, 240 according to the following balanced chemical equation HC0, 2100 NaOH-NaOHO malico LUC02150 65.0 LUC0210 0.400 LHC, 210 1000 LIP CHO malkice, 20 0.400 m.O.O ILH90.240 650 mL KC,O, 20 1 NaOH LEGOKO 1000 ml. GO20 156 L NOOH HGO:20 1 mol CO2KO 0.333 mol NACH maiN OH 1 LNaOH X 1 mol CO2H,0 0.333 mol NOF 2 ml NaOH ADICTOR ist 0.400 0.156 2 0.001 156 7.81 - 100 0.156 0.0781 0.333 30.0 78.1 1.56 10 1000 0,0520 5.20 x 10 1 65.0 molH.CO.2H00 NEOH mi Nu Question 3 of 5 A solution of oxalic acid dihydrato (H.CO, 2H,0) with a known concentration of 0.400 MHCO, ZH, is strated with a 0.333 MNOH solution. How many LNaOH are required to reach the second equivalence point with a starting volume of 65.0 MH.C.0, 2H, according to the following balanced chemical equation: 2 mol NaOH mol H.C.0, 2H,0 H.CO, 2H02 NOOH -- Na.co.4 HO - malce 2150 LHCO, 20 65.0 LHCO,270 0.400 L460 130 1000 m40,0,20 mall,c.0, 2110 BAOK.CO:20 TLK9,0.2,0 65.0 mL. H.C,, 2,0 2 mol NaOH T NHN LHCO 240 1900 6000 1 mol HCO2H0 | 0 1961 NaOH 1 mol 1.0.2H0 0.333 mol NaOH BER it RESET 0.400 0.160 2 0.001 166 7.81 M10 0.156 0.0781 0.333 4 30.0 78.1 1.56 K 10 1000 0.0520 5.20 x 10 1 650 Janda Aw Your Chan ..MY 35 0.400 M., 20.30H. MACH SO, NO C:0,20 HO Dece| MWC LO 1000 LIGGO moko, 20 0.2 RICO, CON TE Olar RECORT 1000 so 6.2006 156 16 TAS 1 2.30 741 1000 6000 5.20 65.0 REDONDONGOLARCOOL Submit Question 3 of 5 A solution of Onic acid hydrate (CO 2HO) with a known concentration of 0.400 MHCO, HO is titrated wana 0.333 MNAOH solution. How many. NaOH are recured to reach the second equivalence point with a starting volume of 65.0 m. H.C.0, 240 according to the following balanced chemical equation HC0, 2100 NaOH-NaOHO malico LUC02150 65.0 LUC0210 0.400 LHC, 210 1000 LIP CHO malkice, 20 0.400 m.O.O ILH90.240 650 mL KC,O, 20 1 NaOH LEGOKO 1000 ml. GO20 156 L NOOH HGO:20 1 mol CO2KO 0.333 mol NACH maiN OH 1 LNaOH X 1 mol CO2H,0 0.333 mol NOF 2 ml NaOH ADICTOR ist 0.400 0.156 2 0.001 156 7.81 - 100 0.156 0.0781 0.333 30.0 78.1 1.56 10 1000 0,0520 5.20 x 10 1 65.0 molH.CO.2H00 NEOH mi Nu Question 3 of 5 A solution of oxalic acid dihydrato (H.CO, 2H,0) with a known concentration of 0.400 MHCO, ZH, is strated with a 0.333 MNOH solution. How many LNaOH are required to reach the second equivalence point with a starting volume of 65.0 MH.C.0, 2H, according to the following balanced chemical equation: 2 mol NaOH mol H.C.0, 2H,0 H.CO, 2H02 NOOH -- Na.co.4 HO - malce 2150 LHCO, 20 65.0 LHCO,270 0.400 L460 130 1000 m40,0,20 mall,c.0, 2110 BAOK.CO:20 TLK9,0.2,0 65.0 mL. H.C,, 2,0 2 mol NaOH T NHN LHCO 240 1900 6000 1 mol HCO2H0 | 0 1961 NaOH 1 mol 1.0.2H0 0.333 mol NaOH BER it RESET 0.400 0.160 2 0.001 166 7.81 M10 0.156 0.0781 0.333 4 30.0 78.1 1.56 K 10 1000 0.0520 5.20 x 10 1 650
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


