Question: hi expert please answer this question asap needed urgently will do thumbs up immediately .thank you The great French chemist Antoine Lavoisier discovered in the
 hi expert please answer this question asap needed urgently will do thumbs up immediately .thank you
hi expert please answer this question asap needed urgently will do thumbs up immediately .thank you
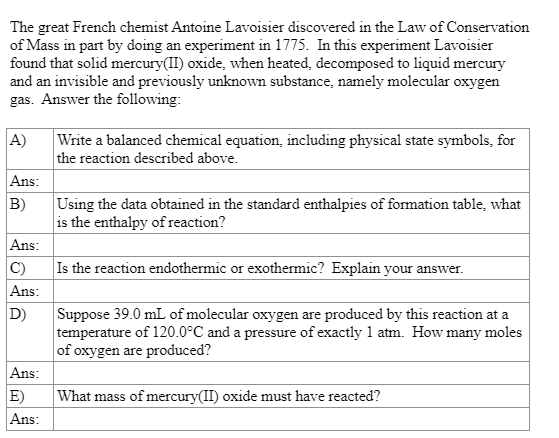
The great French chemist Antoine Lavoisier discovered in the Law of Conservation of Mass in part by doing an experiment in 1775. In this experiment Lavoisier found that solid mercury(II) oxide, when heated, decomposed to liquid mercury and an invisible and previously unknown substance, namely molecular oxygen gas. Answer the following: A) Write a balanced chemical equation, including physical state symbols, for the reaction described above. Ans: B) Using the data obtained in the standard enthalpies of formation table, what is the enthalpy of reaction? Is the reaction endothermic or exothermic? Explain your answer. Ans: C) Ans: D) Suppose 39.0 mL of molecular oxygen are produced by this reaction at a temperature of 120.0C and a pressure of exactly 1 atm. How many moles of oxygen are produced? Ans: E) Ans: What mass of mercury(II) oxide must have reacted
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


