Question: Please write a matlab code to solve the following Exercise 6.16: Temperature and the equilibrium limit The following exothermic, reversible, gas-phase reaction is to be
Please write a matlab code to solve the following
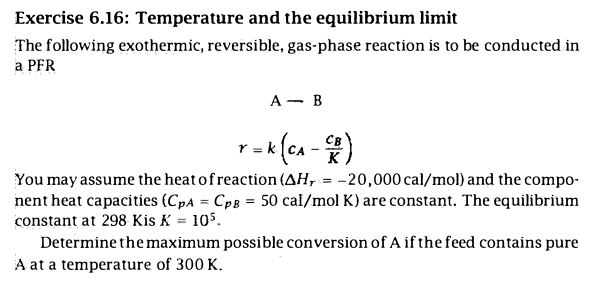
Exercise 6.16: Temperature and the equilibrium limit The following exothermic, reversible, gas-phase reaction is to be conducted in a PFR ABr=k(cAKcB) You may assume the heat of reaction (Hr=20,000cal/mol) and the component heat capacities (CpA=CpB=50cal/molK) are constant. The equilibrium constant at 298KisK=105. Determine the maximum possible conversion of A if the feed contains pure A at a temperature of 300K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


