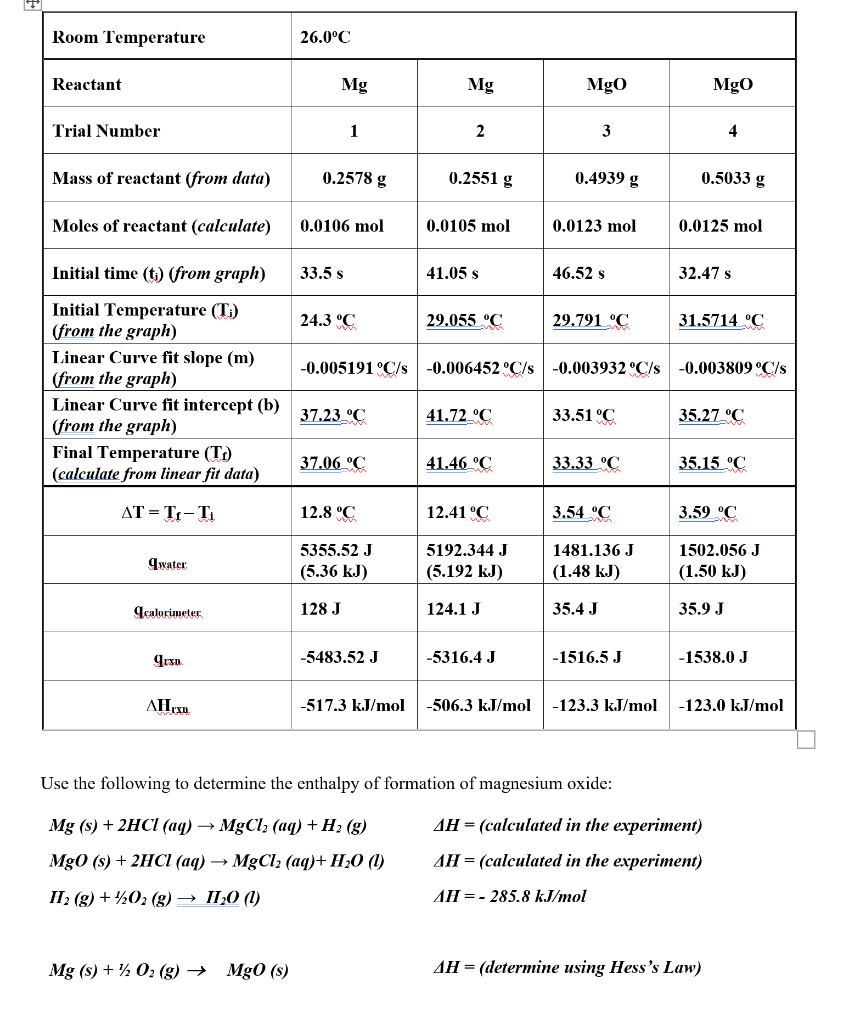
Question: Please write clear the answer to fully understand your hand writing Thank you Use the following to determine the enthalpy of formation of magnesium oxide:

Please write clear the answer to fully understand your hand writing
Thank you
Use the following to determine the enthalpy of formation of magnesium oxide: Mg(s)+2HCl(aq)MgCl2(aq)+H2(g)MgO(s)+2HCl(aq)MgCl2(aq)+H2O(l)H2(g)+1/2O2(g)H2O(l)H=(calculatedintheexperiment)H=(calculatedintheexperiment)H=285.8kJ/mol Mg(s)+1/2O2(g)MgO(s)H=(determineusingHesssLaw) 1. Using the enthalpies of reaction computed in the lab and the value given for the enthalpy of formation of liquid water, show the Hess's Law determination of the enthalpy of formation of solid magnesium oxide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


