Question: I need help with these four questions please and also please write neatly also please do not type thank you 8-41. A 25.0-g sample of


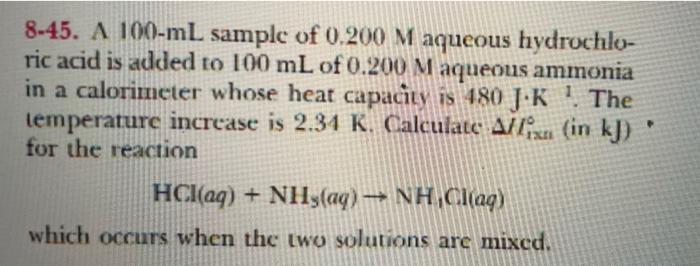
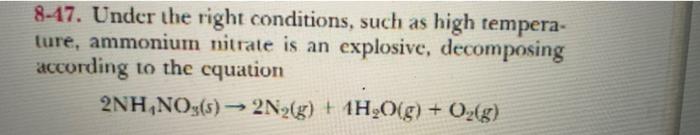

8-41. A 25.0-g sample of copper at 90.0C is placed in 100.0 g of water at 20.0C. The copper and water quickly come to the same temperature by the process of heat transfer from copper to water. Calculate the final tem- perature of the water in C. The heat capacity of copper is 24.5 J K mol-!. 8-43. A 1.00-kg block of aluminum metal (Cp = 24.2 J.K--moll) at 500C is placed in contact with a 1.00-kg block of copper (Cp = 24.5 J-K--mol-!) at 10C. What will be thc final temperature (in "C) of the two blocks? Assume that no heat is lost to the surround- ings. 8-45. A 100-mL sample of 0.200 M aqueous hydrochlo- ric acid is added to 100 mL of 0.200 Maqueous ammonia in a calorimeter whose heat capacity is 180 JK ! The temperature increase is 2.34 K. Calculate Alix (in kJ) for the reaction HCl(aq) + NH3(aq) NH,Clag) which occurs when the two solutions are mixed. 8-47. Under the right conditions, such as high tempera- ture, ammonium nitrate is an explosive, decomposing according to the cquation 2NH,NO () 2N2(g) + 1H2O(g) + O2(g) A 1.00-g sample of NH NO3 is detonated in a calorime- ter whose heat capacity is 4.92 kJ.K*'. The temperature increase is 0.300 K Calculate the heat of reaction for the decomposition of 1,00 kg of aminonium nitrate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


