Question: Pleaseee answer both questions :((( Thank youuuu Question 1 Question 2 The product of Example 6.1 undergoes a saponification reaction that hydrolyzes the ethyl group
Pleaseee answer both questions :(((
Thank youuuu
Question 1
Question 2
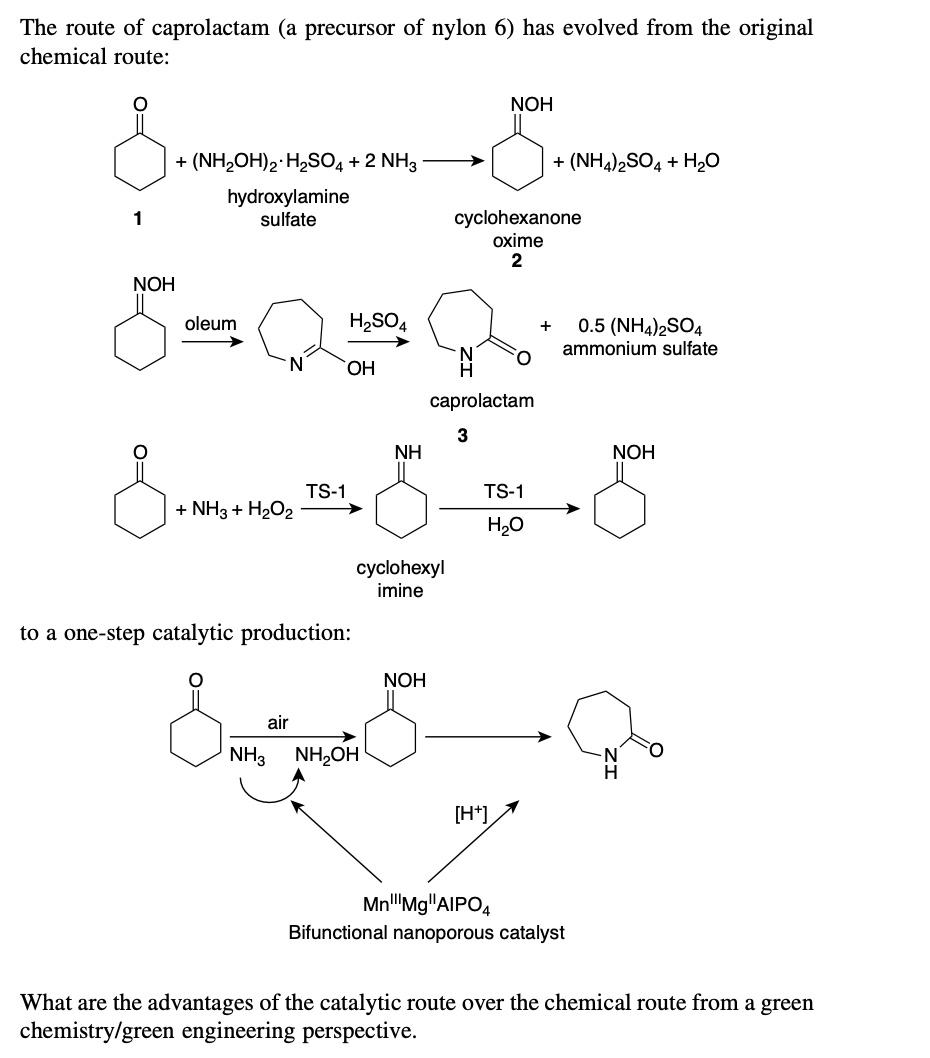
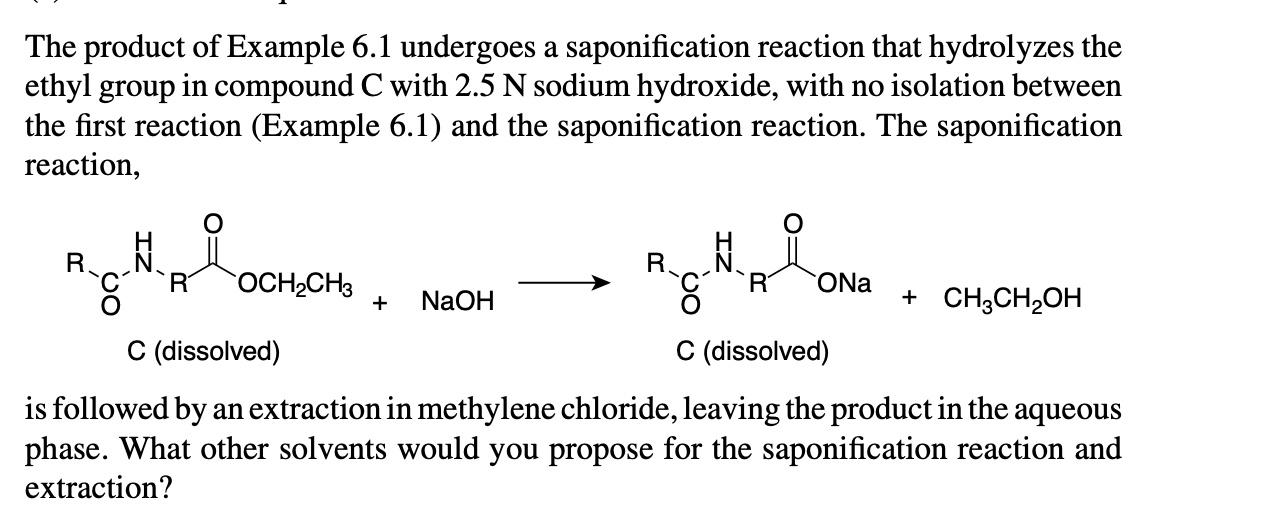
The product of Example 6.1 undergoes a saponification reaction that hydrolyzes the ethyl group in compound C with 2.5N sodium hydroxide, with no isolation between the first reaction (Example 6.1) and the saponification reaction. The saponification reaction, is followed by an extraction in methylene chloride, leaving the product in the aqueous phase. What other solvents would you propose for the saponification reaction and extraction? The route of caprolactam (a precursor of nylon 6) has evolved from the original chemical route: +(NH2OH)2H2SO4+2NH3(NH4)2SO4+H2O hydroxylamine sulfate cyclohexanone 2oxime caprolactam to a one-step catalytic production: What are the advantages of the catalytic route over the chemical route from a green chemistry/green engineering perspective
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


