Question: pleaseee help ASAP 3. The reversible-liquid phase reaction, A+2B 3C, is carried out at a isothermal CSTR, where CAo-CBo= 2M and Cco-0. Please estimate the
pleaseee help ASAP
3. The reversible-liquid phase reaction, A+2B 3C, is carried out at a isothermal CSTR, where CAo-CBo= 2M and Cco-0.
Please estimate the -TA,ne as X=1/3, where Kg = 0.5 L*sec/mol, K= 0.5. The reaction order of A, B, and C are 1,1, and
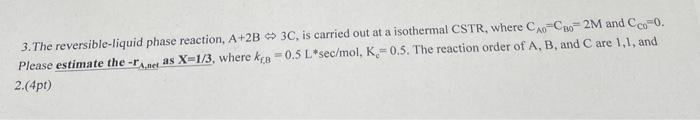
3. The reversible-liquid phase reaction, A+2B3C, is carried out at a isothermal CSTR, where CA0=CBO=2M and CCO=0. Please estimate the rA,nec as X=1/3, where kf,B=0.5Lsec/mol,Kc=0.5. The reaction order of A,B, and C are 1,1 , and 2.(4pt)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


