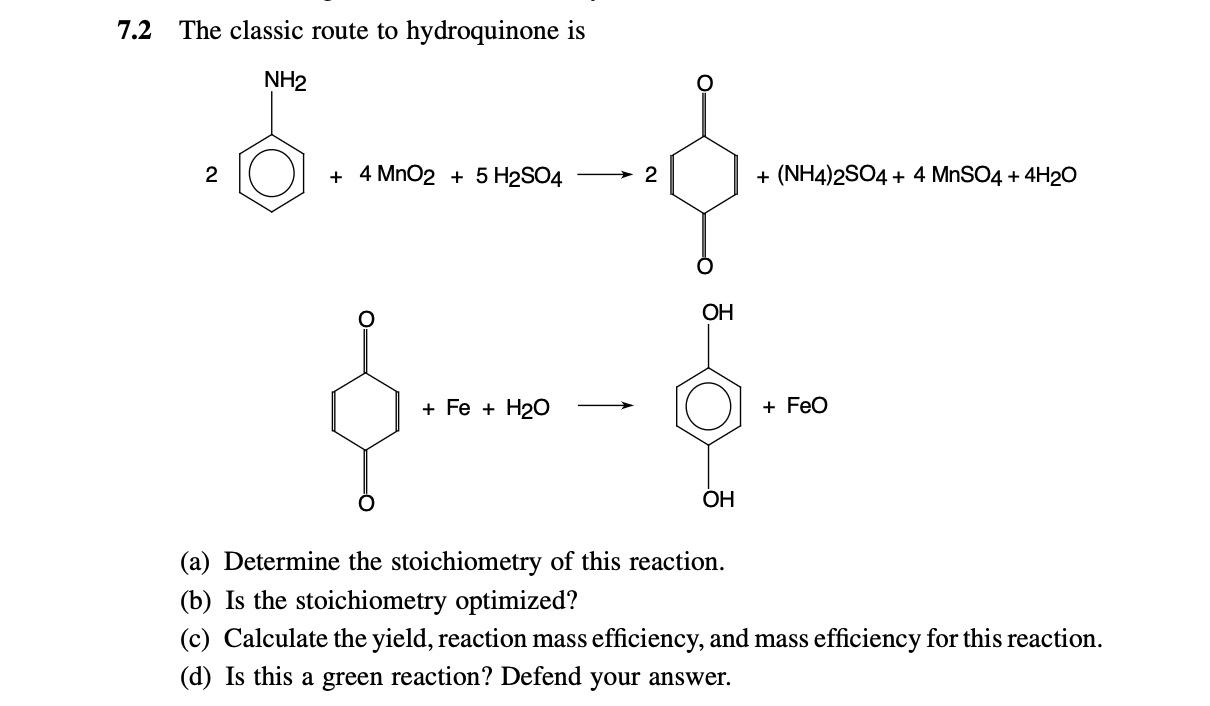
Question: Pleaseeeee help me with this qu please answer all parts 7.2 The classic route to hydroquinone is 2 +(NH4)2SO4+4MnSO4+4H2O (a) Determine the stoichiometry of this
Pleaseeeee help me with this qu
please answer all parts
7.2 The classic route to hydroquinone is 2 +(NH4)2SO4+4MnSO4+4H2O (a) Determine the stoichiometry of this reaction. (b) Is the stoichiometry optimized? (c) Calculate the yield, reaction mass efficiency, and mass efficiency for this reaction. (d) Is this a green reaction? Defend your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


