Question: pls answer all At pH7, the side chain hydroxyl group of a free serine amino acid has , allowing it to a hydrogen bond of


pls answer all
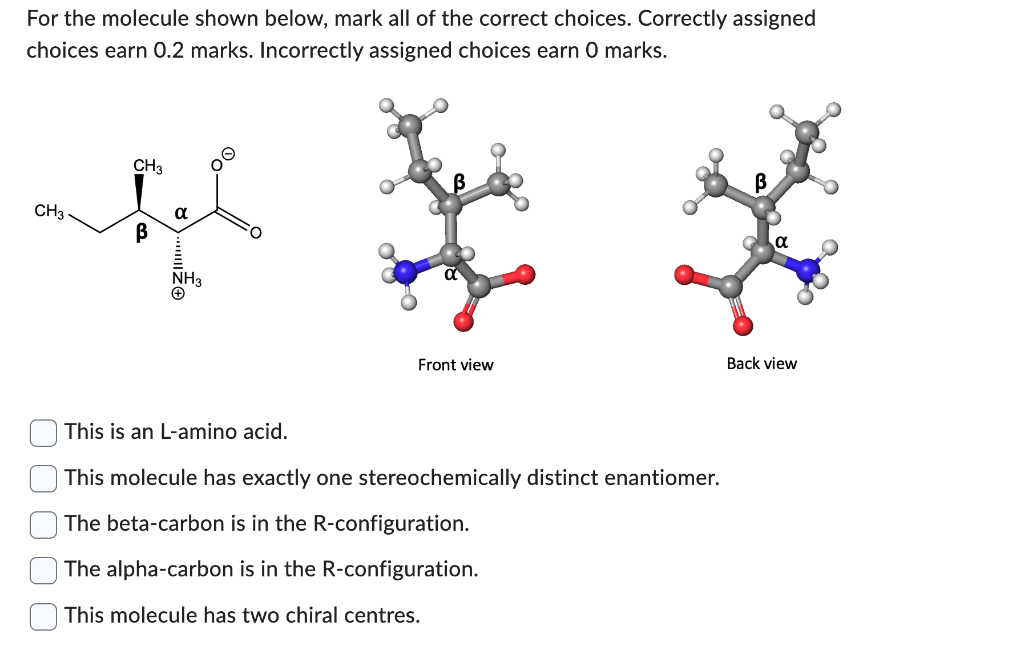
At pH7, the side chain hydroxyl group of a free serine amino acid has , allowing it to a hydrogen bond of the amide group in the side chain of asparagine. (For full marks, please choose ALL answers that are correct. You will earn 0.25 marks for each answer identified as either true (selected) or false (not selected.) a hydrogen atom / donate / to the lone pair a lone pair / accept / from the lone pair a hydrogen atom / accept / from the hydrogen atom a lone pair / accept / from the hydrogen atom For the molecule shown below, mark all of the correct choices. Correctly assigned choices earn 0.2 marks. Incorrectly assigned choices earn 0 marks. This is an L-amino acid. This molecule has exactly one stereochemically distinct enantiomer. The beta-carbon is in the R-configuration. The alpha-carbon is in the R-configuration. This molecule has two chiral centres. Which of the following statements MUST BE TRUE if a reaction is spontaneous at room temperature? Each correctly assigned choice earns 0.25 marks. When heat is absorbed (i.e., when enthalpy in the system increases), the change in entropy in the system must always be positive. The change in the entropy of the system must always be positive. When heat is released (i.e., when enthalpy decreases in the system), the change in entropy in the system must always be positive. The change in free energy must always be negative. Question 3 (1 point) What is the change in free energy for a reaction at 27 degrees C(300K) with deltaH=10kJ/mol and delta- S=30J/mol/K ? Is this reaction spontaneous? +1kJ/mol; no, it is not spontaneous +1kJ/mol; yes, it is spontaneous 1kJ/mol; no, it is not spontaneous 1kJ/mol; yes, it is spontaneous
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


