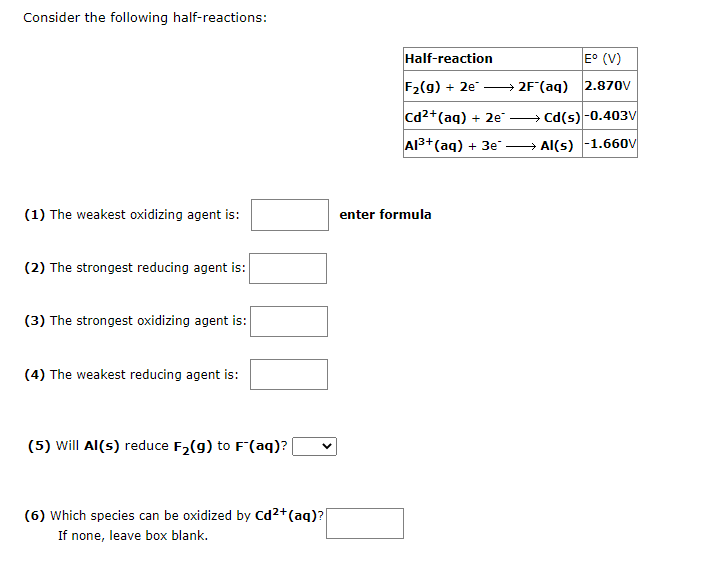
Question: pls answer the 2 items will upvote Consider the following half-reactions: (1) The weakest oxidizing agent is: enter formula (2) The strongest reducing agent is:
pls answer the 2 items will upvote
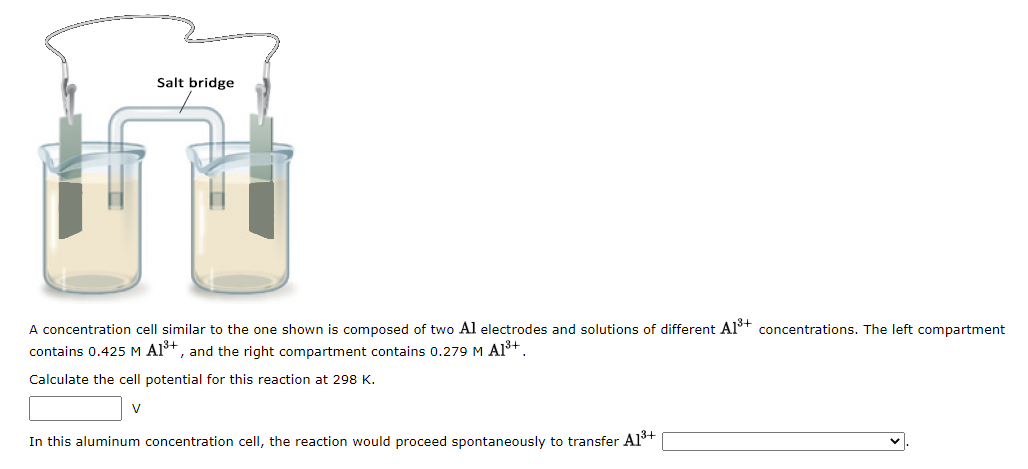
Consider the following half-reactions: (1) The weakest oxidizing agent is: enter formula (2) The strongest reducing agent is: (3) The strongest oxidizing agent is: (4) The weakest reducing agent is: (5) Will Al(s) reduce F2(g) to F(aq) ? (6) Which species can be oxidized by Cd2+(aq) ? If none, leave box blank. A concentration cell similar to the one shown is composed of two Al electrodes and solutions of different Al3+ concentrations. The left compartment contains 0.425MAl3+, and the right compartment contains 0.279MAl3+. Calculate the cell potential for this reaction at 298K. V In this aluminum concentration cell, the reaction would proceed spontaneously to transfer Al3+
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


