Question: Consider the following half - reactions: ( 1 ) The strongest oxidizing agent is: enter formula ( 2 ) The weakest oxidizing agent is: (
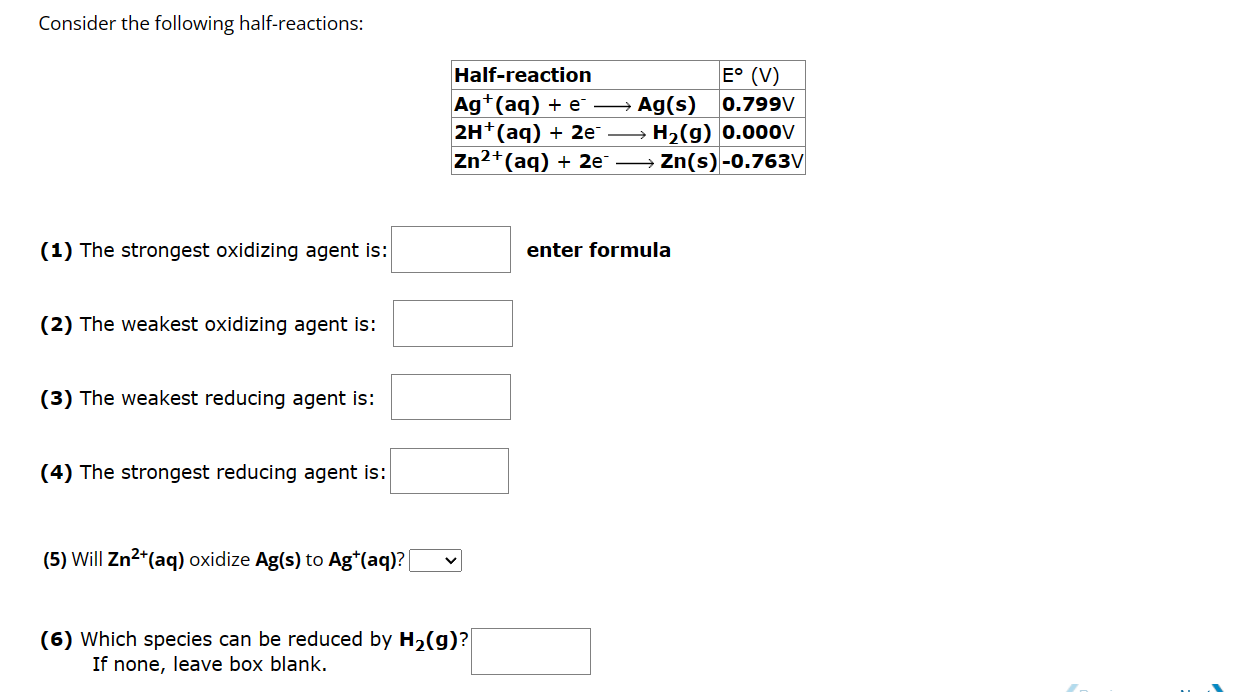
Consider the following halfreactions:
The strongest oxidizing agent is:
enter formula
The weakest oxidizing agent is:
The weakest reducing agent is:
The strongest reducing agent is:
Will oxidize to
Which species can be reduced by
If none, leave box blank.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


