Question: Pls can you solve step by step with a calculation and GRAPH, it is very important. Thanks. Q 1 : An isothermal, irreversible gas -
Pls can you solve step by step with a calculation and GRAPH, it is very important. Thanks.
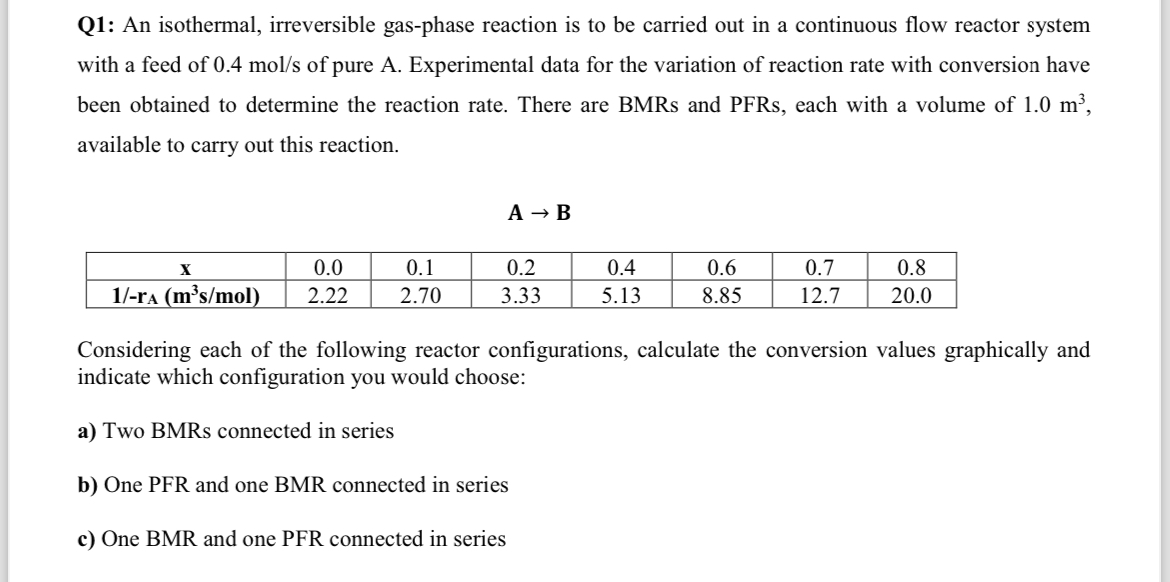
Q: An isothermal, irreversible gasphase reaction is to be carried out in a continuous flow reactor system with a feed of of pure A Experimental data for the variation of reaction rate with conversion have been obtained to determine the reaction rate. There are BMRs and PFRs each with a volume of available to carry out this reaction.
table
Considering each of the following reactor configurations, calculate the conversion values graphically and indicate which configuration you would choose:
a Two BMRs connected in series
b One PFR and one BMR connected in series
c One BMR and one PFR connected in series
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


