Question: pls help 3 100 Element X has three naturally occurring isotopes as shown in the table below. Based on the data in the table, what
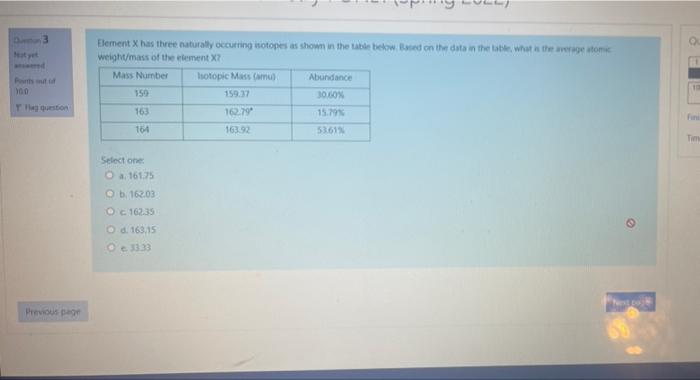
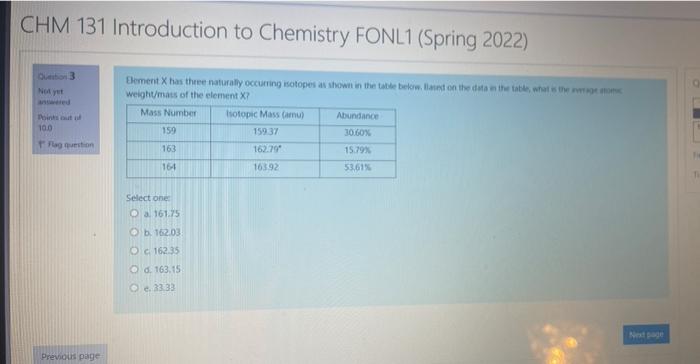
3 100 Element X has three naturally occurring isotopes as shown in the table below. Based on the data in the table, what is there om weight/mass of the element ? Mass Number Isotopic Mass Com Abundance 159.37 30.10% 163 162.79 15.793 164 163.32 53,61% T Select one 161.25 e b. 16203 O C16235 6.163.15 @1333 Previous page CHM 131 Introduction to Chemistry FONL1 (Spring 2022) Do 3 Noy Doinut 100 Element X has the naturally occurring isotopes as shown in the table below lanet on the data is the title what we are weight/mass of the element X? Mass Number Isotopic Mass Caru) Abundance 159 15937 30,60% 162.79 15.79% 16 16392 53,61% Flag veston 163 Select one 161.75 162.03 Oc 162.35 d. 163.15 33.33 Note Previous page
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


