Question: pls help ASAP, multiple choice questions: no need to show full computation I am just checking my work The K0 of benzoic acid (C6H5COOH) is
pls help ASAP, multiple choice questions: no need to show full computation I am just checking my work 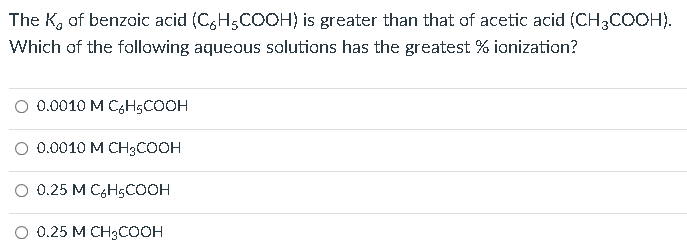
The K0 of benzoic acid (C6H5COOH) is greater than that of acetic acid (CH3COOH). Which of the following aqueous solutions has the greatest \% ionization? 0.0010MC6H5COOH0.0010MCH3COOH0.25MC6H5COOH0.25MCH3COOH
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


