Question: A Laboratory conducted a test for simple oxidation reaction SO2 + 12 02 SO3 in a differential fixed bed reactor. The reaction was conducted at
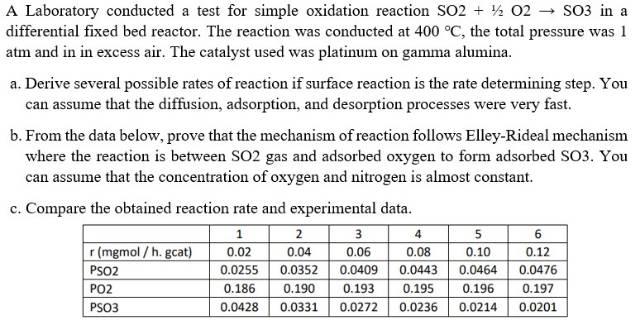
A Laboratory conducted a test for simple oxidation reaction SO2 + 12 02 SO3 in a differential fixed bed reactor. The reaction was conducted at 400 C, the total pressure was 1 atm and in in excess air. The catalyst used was platinum on gamma alumina. a. Derive several possible rates of reaction if surface reaction is the rate determining step. You can assume that the diffusion, adsorption, and desorption processes were very fast. b. From the data below, prove that the mechanism of reaction follows Elley-Rideal mechanism where the reaction is between SO2 gas and adsorbed oxygen to form adsorbed SO3. You can assume that the concentration of oxygen and nitrogen is almost constant. c. Compare the obtained reaction rate and experimental data. r(mgmol / h. gcat) PSO2 PO2 PSO3 1 0.02 0.0255 0.186 0.0428 2 0.04 0.0352 0.190 0.0331 3 0.06 0.0409 0.193 0.0272 4 0.08 0.0443 0.195 0.0236 5 0.10 0.0464 0.196 0.0214 6 0.12 0.0476 0.197 0.0201
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


