Question: pls help Calculate solution concentration A solution consists of 44.3 g of ammonia, NH3, and 103.7 g water. (a) Calculate the weight percent, the molality,

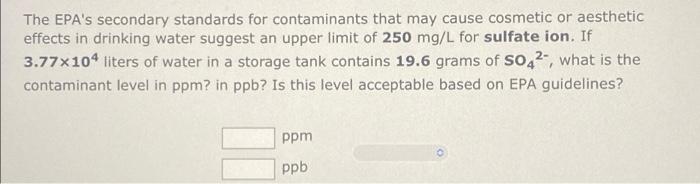
Calculate solution concentration A solution consists of 44.3 g of ammonia, NH3, and 103.7 g water. (a) Calculate the weight percent, the molality, and the mole fraction of NH3 in the solution. % weight percent = molality = mole fraction = m (b) The solution volume is 166 ml. Calculate the molarity of NH3 in the solution. molarity M The EPA's secondary standards for contaminants that may cause cosmetic or aesthetic effects in drinking water suggest an upper limit of 250 mg/L for sulfate ion. If 3.77x104 liters of water in a storage tank contains 19.6 grams of s042, what is the contaminant level in ppm? in ppb? Is this level acceptable based on EPA guidelines? ppm ppb
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


