Question: Pls help I dont understand how to get the correct answer! consider a hypothetical reaction: A22A The concentration of A2 was measured over the first
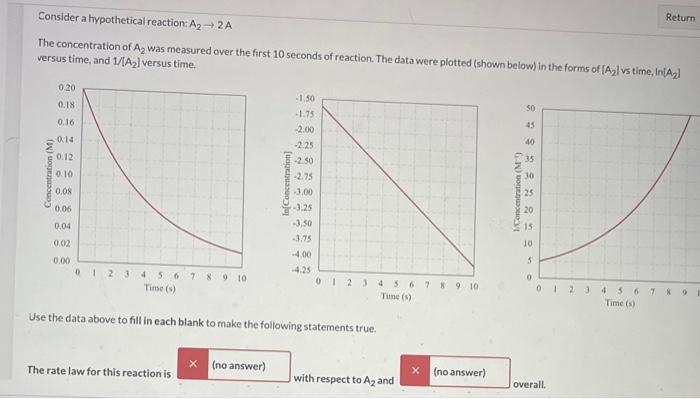

consider a hypothetical reaction: A22A The concentration of A2 was measured over the first 10 seconds of reaction. The data were plotted (shown below) in the forms of [A2] l vs time, in A2] versus time, and 1/A2) versus time. Use the data above to fill in each blank to make the following statements true. The rate law for this reaction is with respect to A2 and overall. Use the data above to fill in each blank to make the following statements true. The rate law for this reaction is with respect to A2 and overall. Correct Answer: first-order Correct Answer: first-order The rate constant for this reaction is Correct Answer: 0.251/s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


