Question: pls help identify the product and answer the questions! 4) What will be different between the IR of the starting materials and product? Procedure 1.

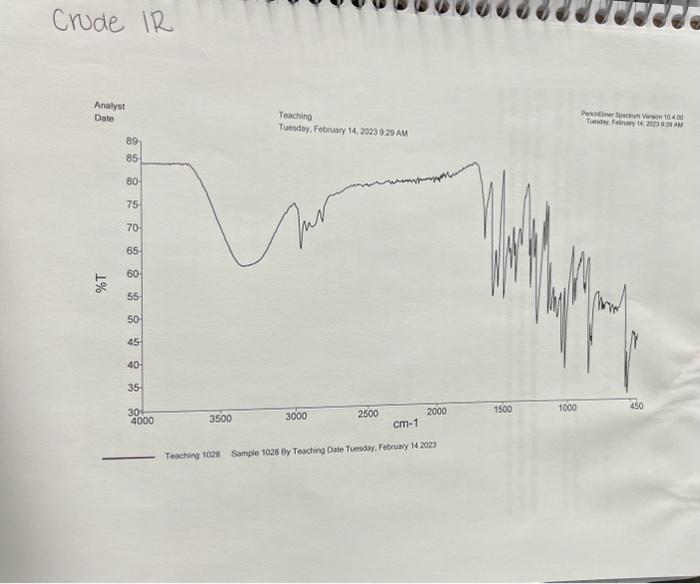
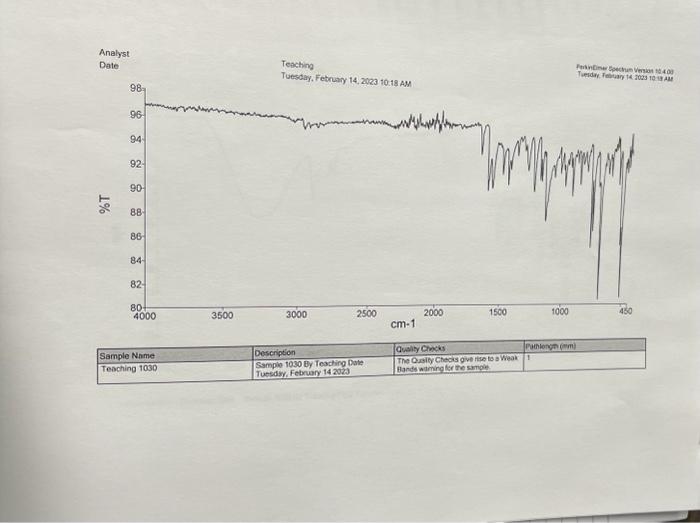
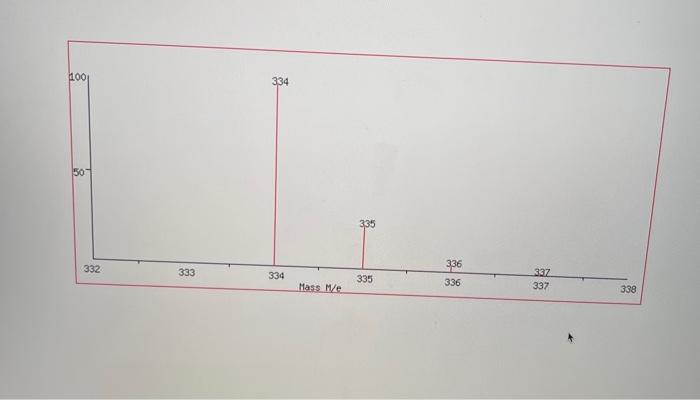
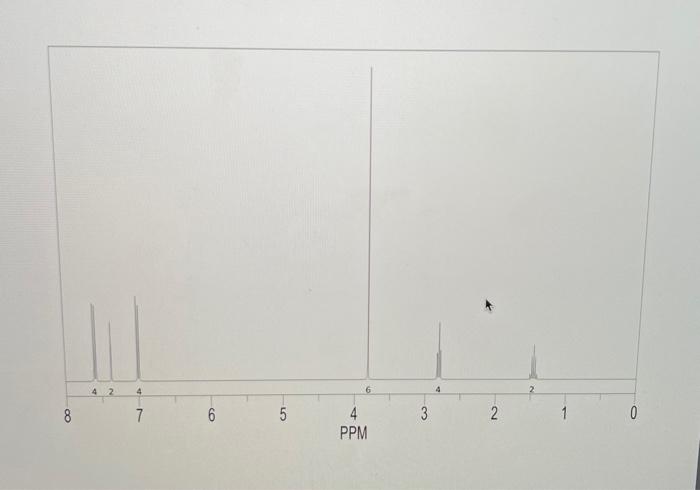
4) What will be different between the IR of the starting materials and product? Procedure 1. Reaction Set-up Combine the unknown aldehyde and ketone you've been assigned in 95% Ethanol (8mL) and ensure everything has dissolved (additional EtOH may be required - ask your TA) in an appropriately-sized round bottom flask. Then slowly add the 6MNaOH ias solution, followed by stirring for approximately 1520 minutes. The reaction is complete when precipitate stops forming. If precipitate is still forming, then let the reaction continue stirring until it appears to complete. Alternatively, if there is no demonstrable amount of solid, then you may need to reflux the reaction for an additional 15 minutes. 2. Isolation and Purificotion of Product After the reaction is complete, cool the flask to room temperature if necessary, and then place the flask in an ice bath for 10 minutes. Vacuum filter the product. Rinse the product with between 24mL of cold 95% ethanol, then allow to briefly dry on the filter. Determine the crude mass, and save approximately 10 mg of product for melting point and IR analysis. After saving 10mg of crude material for analysis, perform recrystallization from 95% ethanol- remember to use two separate beakers/Erlenmeyers for this purpose, one for recrystallization, and one to keep your solvent at reflux. Also, remember to add solvent slowly to ensure you do not make the solution too dilute to effectively recrystallize, or you will have to evaporate your solvent and start over. Once all of the material has dissolved, allow the flask to cool slowly to room temperature, and then add to an ice bath if necessary. Use filtration to separate the mother liquor from the precipitate, and then dry on the filter. Make sure to determine the purified mass. 3. Analysis of Product You should have determined the mass of your product both before and after purification. You should take IR of both the crude and purified products, as well as the melting points. You will be provided with an 14 NMR spectrum as well as a mass spectrum of your purified product on Blackboard. Questions to Address in Your Report 1) From the spectrai data (2 H,IR,MS) you were given, identify the structure of your product. Explain why you chose your particular product based on the spectroscopic data. You will not receive full marks for determination of the unknown unless you explain why. 2) Would you suggest that your purified compound is greater than 95% pure based on the IR spectrum and melting point? Postlab Questions 1) You performed this reaction with excess aldehyde ( 2.5 equiv.) - what do you think would be the outcome of using the ketone in slight excess (1.2 equiv.)? 2) Draw the mechanism for a base catalyzed aldol condensation between acetophenone and benzaldehyde (the aromatic can be abbreviated Ph in both cases). Make sure to consider which steps are reversible, if any, and which aren't. 3) The reaction you've performing is formerly called a cross aldol condensation. What makes this reaction a crossed aldol condensation, and why is it easier to achieve a good yield of a single product using these specific reagents (aromatic aldehyde and symmetrical ketone) than many other aldol condensation reactions? Crude IR 4) What will be different between the IR of the starting materials and product? Procedure 1. Reaction Set-up Combine the unknown aldehyde and ketone you've been assigned in 95% Ethanol (8mL) and ensure everything has dissolved (additional EtOH may be required - ask your TA) in an appropriately-sized round bottom flask. Then slowly add the 6MNaOH ias solution, followed by stirring for approximately 1520 minutes. The reaction is complete when precipitate stops forming. If precipitate is still forming, then let the reaction continue stirring until it appears to complete. Alternatively, if there is no demonstrable amount of solid, then you may need to reflux the reaction for an additional 15 minutes. 2. Isolation and Purificotion of Product After the reaction is complete, cool the flask to room temperature if necessary, and then place the flask in an ice bath for 10 minutes. Vacuum filter the product. Rinse the product with between 24mL of cold 95% ethanol, then allow to briefly dry on the filter. Determine the crude mass, and save approximately 10 mg of product for melting point and IR analysis. After saving 10mg of crude material for analysis, perform recrystallization from 95% ethanol- remember to use two separate beakers/Erlenmeyers for this purpose, one for recrystallization, and one to keep your solvent at reflux. Also, remember to add solvent slowly to ensure you do not make the solution too dilute to effectively recrystallize, or you will have to evaporate your solvent and start over. Once all of the material has dissolved, allow the flask to cool slowly to room temperature, and then add to an ice bath if necessary. Use filtration to separate the mother liquor from the precipitate, and then dry on the filter. Make sure to determine the purified mass. 3. Analysis of Product You should have determined the mass of your product both before and after purification. You should take IR of both the crude and purified products, as well as the melting points. You will be provided with an 14 NMR spectrum as well as a mass spectrum of your purified product on Blackboard. Questions to Address in Your Report 1) From the spectrai data (2 H,IR,MS) you were given, identify the structure of your product. Explain why you chose your particular product based on the spectroscopic data. You will not receive full marks for determination of the unknown unless you explain why. 2) Would you suggest that your purified compound is greater than 95% pure based on the IR spectrum and melting point? Postlab Questions 1) You performed this reaction with excess aldehyde ( 2.5 equiv.) - what do you think would be the outcome of using the ketone in slight excess (1.2 equiv.)? 2) Draw the mechanism for a base catalyzed aldol condensation between acetophenone and benzaldehyde (the aromatic can be abbreviated Ph in both cases). Make sure to consider which steps are reversible, if any, and which aren't. 3) The reaction you've performing is formerly called a cross aldol condensation. What makes this reaction a crossed aldol condensation, and why is it easier to achieve a good yield of a single product using these specific reagents (aromatic aldehyde and symmetrical ketone) than many other aldol condensation reactions? Crude IR
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


