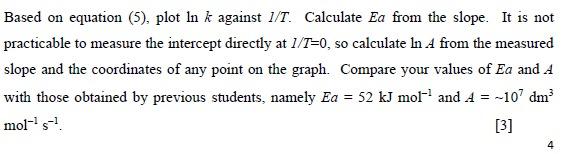
Question: [[[Pls plot and calculate]]] Mixtures 1. 6 and 7 k Temperature In k 0C 9.325x10-4 -6.98 RT 3.925*10-3 -5.54 35 C 12.25x10-3 -4.40 Based on
![[[[Pls plot and calculate]]] Mixtures 1. 6 and 7 k Temperature](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8f5dd44347_90066f8f5dced6e4.jpg)

[[[Pls plot and calculate]]]
Mixtures 1. 6 and 7 k Temperature In k 0C 9.325x10-4 -6.98 RT 3.925*10-3 -5.54 35 C 12.25x10-3 -4.40 Based on equation (5), plot In k against 1/T. Calculate Ea from the slope. It is not practicable to measure the intercept directly at 1/1=0, so calculate In A from the measured slope and the coordinates of any point on the graph. Compare your values of Ea and A with those obtained by previous students, namely Ea = 52 kJ mol and A = --107 dm3 mol-5-7 [3] A (pre-exponential factor) and Ea (activation energy) are both independent of temperature. Note that A has the same units as k. Taking logarithms, we obtain In k = In A - Ea/RT (5) so that a graph of In k against 1/T should be linear, with slope = - Ea/R and intercept = In 4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


