Question: pls solve all pages asap Part I. Classify the Structures Using VSEPR Theory A. Consider the following structure: B. Consider the following structure: C. Consider
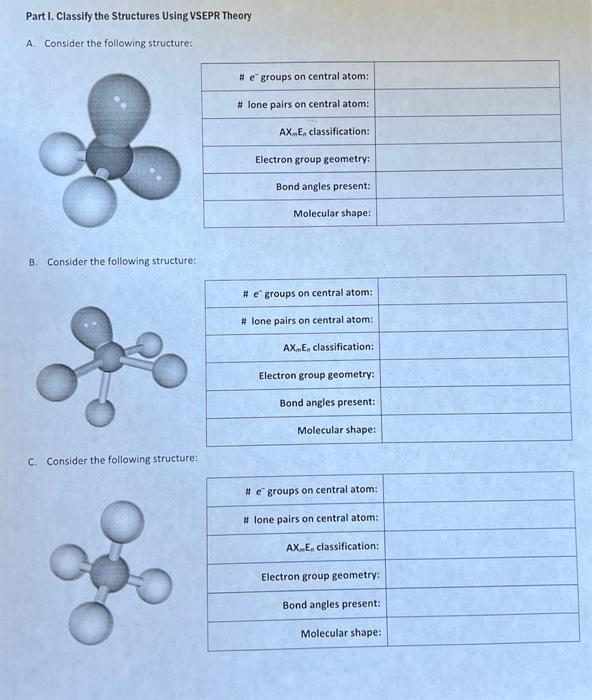
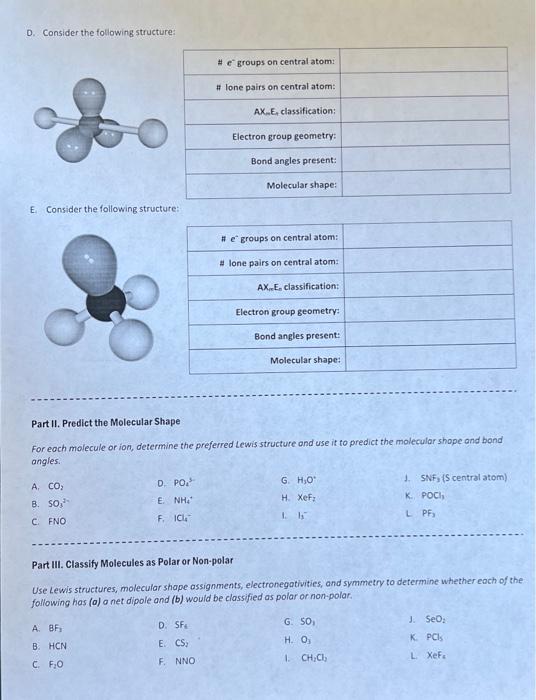
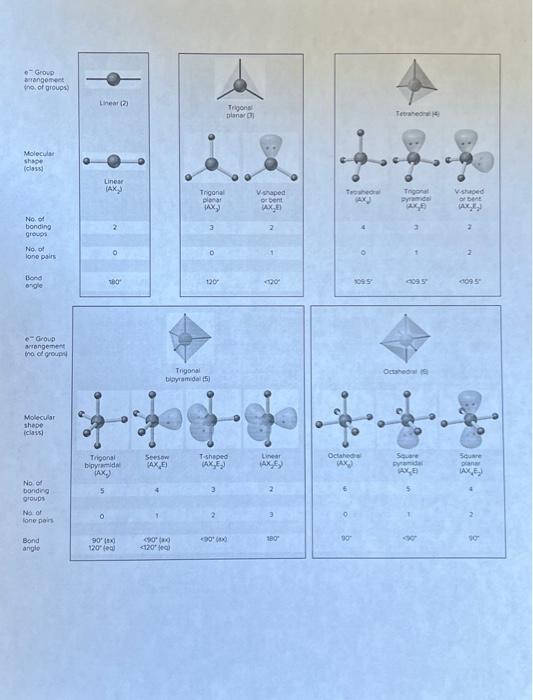
Part I. Classify the Structures Using VSEPR Theory A. Consider the following structure: B. Consider the following structure: C. Consider the following structure: D. Consider the foliowing structure: E. Consider the following structure: Part II. Predict the Molecular Shape For eoch molecule or ion, determine the preferred Lewis structure and use it to predict the molecular shope and bond angles. A. CO2 D. PO43 G. H3O+ d. SNFy \{S central atom) B. SO32 E. NH4+ H. Xefz K. POCls. c. FNO F. ICl4 1. Is L. PFy Part III. Classify Molecules as Polar or Non-polar Use Lewis structures, molecular shope assignments, electronegativities, and symmetry to determine whether each of thefollowing has (a) a net dipole and (b) would be classified as polar or non-polar. A. BFi D. SFi G. 5O3 J. SeO2 B. HCN E. CS2 H. O3 K. PCls C. F2O F. NNO 1. CH2Cl4 L. Xefr Periodic Table of the Elements @0.99@creativo
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


