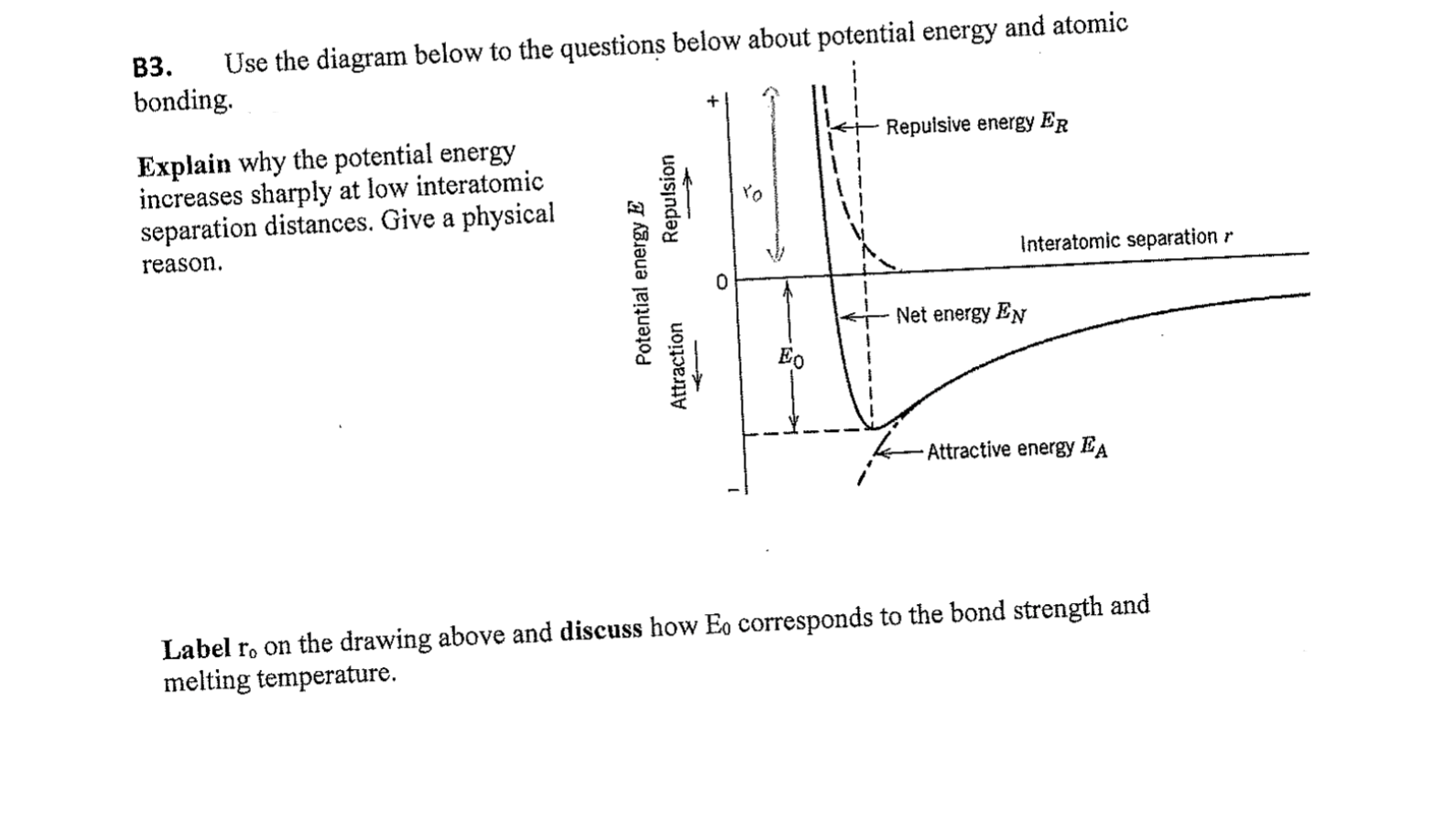
Question: PLS, solve it step by step. B3. Use the diagram below to the questions below about potential energy and atomic bonding Repuisive energy ER Explain
PLS, solve it step by step.
B3. Use the diagram below to the questions below about potential energy and atomic bonding Repuisive energy ER Explain why the potential energy increases sharply at low interatomic separation distances. Give a physical Repulsion Po Interatomic separation r reason. Potential energy E Attraction Net energy En Attractive energy EA Label ro on the drawing above and discuss how E, corresponds to the bond strength and melting temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


