Question: Pls step by step solution,clearly. I need it pls step by step. The ethanol-water solution is poured into a distillation column operating at atmospheric pressure
Pls step by step solution,clearly. I need it pls step by step.
The ethanol-water solution is poured into a distillation column operating at atmospheric pressure (101.3 kPa) with a flow rate of 1000 kg/h. It is distilled by feeding and in the process a head product containing 90% ethanol and a base containing 95% water by weight product is obtained. The enthalpy of the feed stream entering the column as a vapor-liquid mixture is 1000 kJ/kg. An entire condenser is connected to the column, and the enthalpy of the liquid phase leaving the condenser is 100 kJ/kg. Saturated water vapor, used for heating and having an absolute pressure of 476 kPa, enters the column from the bottom of the lowest shelf. It is fed directly and the minimum riflux ratio is 1. Since the riflux ratio is 1.5 under the processing conditions, Calculate the amount of water vapor required for the process using the enthalpy-composition diagram.
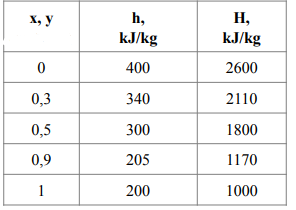
The equilibrium and enthalpy values of the ethanol-water system at 101.3 kPa are in the table.
x and y % weight:
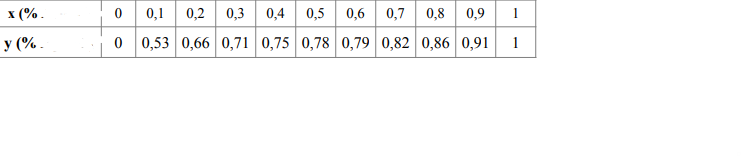
\begin{tabular}{|c|c|c|} \hline x,y & h,kJ/kg & H,kJ/kg \\ \hline 0 & 400 & 2600 \\ \hline 0,3 & 340 & 2110 \\ \hline 0,5 & 300 & 1800 \\ \hline 0,9 & 205 & 1170 \\ \hline 1 & 200 & 1000 \\ \hline \end{tabular} \begin{tabular}{c|c|c|c|c|c|c|c|c|c|c|c|} x(%. & 0 & 0,1 & 0,2 & 0,3 & 0,4 & 0,5 & 0,6 & 0,7 & 0,8 & 0,9 & 1 \\ \hline y(% & 0 & 0,53 & 0,66 & 0,71 & 0,75 & 0,78 & 0,79 & 0,82 & 0,86 & 0,91 & 1 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


