Question: plz asap solve withh full ans Question 2 a) A Nz(1)/CO2(2) gaseous mixture which contains 0.04 mol of N2 and 0.04 mol of CO2 in
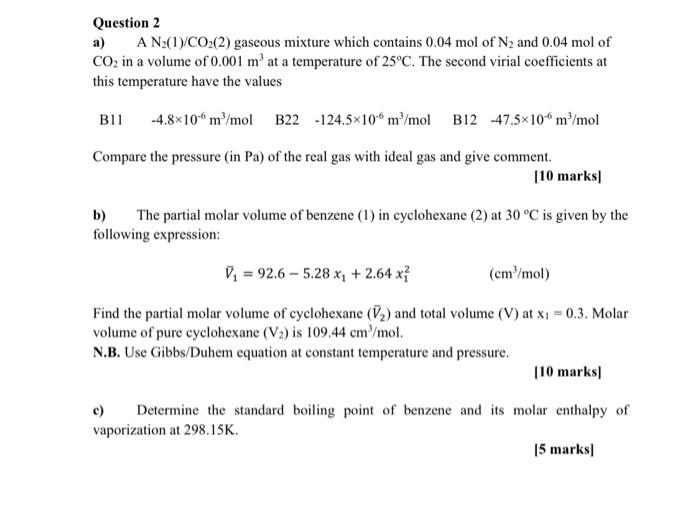
Question 2 a) A Nz(1)/CO2(2) gaseous mixture which contains 0.04 mol of N2 and 0.04 mol of CO2 in a volume of 0.001 mat a temperature of 25C. The second virial coefficients at this temperature have the values B11 -4.8*100m/mol B22-124.5x10 m /mol B12 47.5x10 m/mol Compare the pressure (in Pa) of the real gas with ideal gas and give comment. [10 marks) b) The partial molar volume of benzene (1) in cyclohexane (2) at 30C is given by the following expression: V = 92.6 - 5.28 x: + 2.64 x? (cm /mol) Find the partial molar volume of cyclohexane (72) and total volume (V) at x1 = 0.3. Molar volume of pure cyclohexane (V2) is 109.44 cm mol. N.B. Use Gibbs/Duhem equation at constant temperature and pressure. 110 marks) c) Determine the standard boiling point of benzene and its molar enthalpy of vaporization at 298.15K 15 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


