Question: Please solve it in one hour i will surely thumbs up you please solve it Question 2 a) A Nz(1)/CO2(2) gaseous mixture which contains 0.04
Please solve it in one hour i will surely thumbs up you please solve it
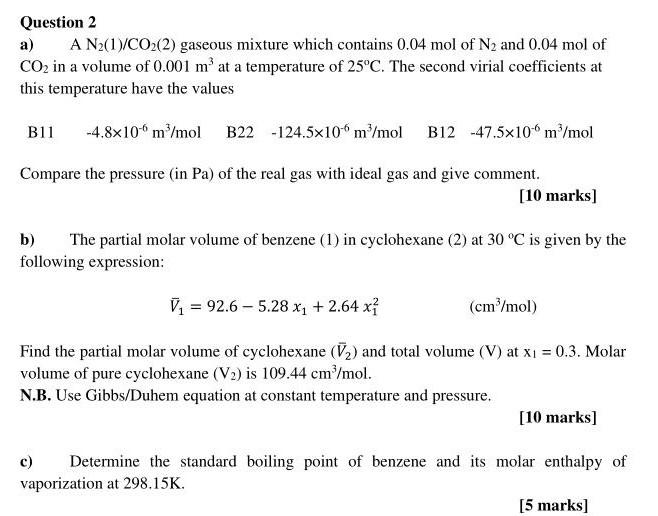
Question 2 a) A Nz(1)/CO2(2) gaseous mixture which contains 0.04 mol of N2 and 0.04 mol of CO2 in a volume of 0.001 m'at a temperature of 25C. The second virial coefficients at this temperature have the values B11 -4.8x10 m/mol B22-124.5x10 m/mol B12 -47.5x10 m /mol Compare the pressure (in Pa) of the real gas with ideal gas and give comment. [10 marks] b) The partial molar volume of benzene (1) in cyclohexane (2) at 30C is given by the following expression: V = 92.6 - 5.28 x1 +2.64 x (cm /mol) Find the partial molar volume of cyclohexane () and total volume (V) at x1 = 0.3. Molar volume of pure cyclohexane (V) is 109.44 cm /mol. N.B. Use Gibbs/Duhem equation at constant temperature and pressure. [10 marks] c) Determine the standard boiling point of benzene and its molar enthalpy of vaporization at 298.15K. [5 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


