Question: plz help from a to g Unit 5, Activity 6 Balancing Redox Reactions Worksheet Balancing Redox Reactions using oxidation number method worksheet Balance the following
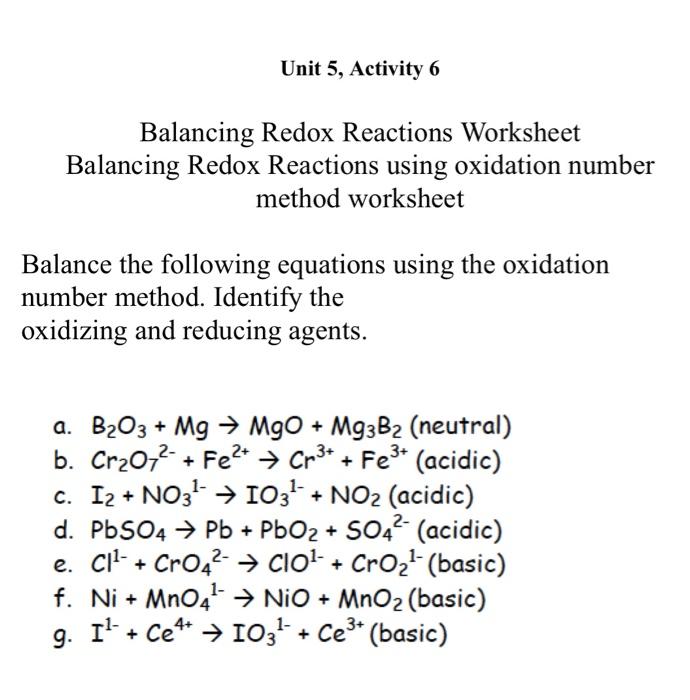
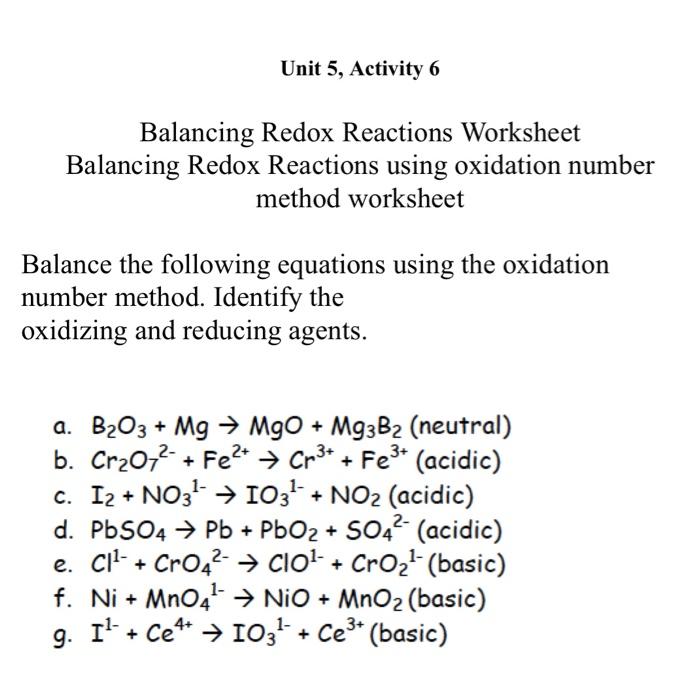
Unit 5, Activity 6 Balancing Redox Reactions Worksheet Balancing Redox Reactions using oxidation number method worksheet Balance the following equations using the oxidation number method. Identify the oxidizing and reducing agents. a. B2O3+MgMgO+Mg3B2 (neutral) b. Cr2O72+Fe2+Cr3++Fe3+ (acidic) c. I2+NO31IO31+NO2 (acidic) d. PbSO4Pb+PbO2+SO42 (acidic) e. Cl1+CrO42ClO1+CrO21 (basic) f. Ni+MnO41NiO+MnO2 (basic) g. I1+Ce4+IO31+Ce3+ (basic) Unit 5, Activity 6 Balancing Redox Reactions Worksheet Balancing Redox Reactions using oxidation number method worksheet Balance the following equations using the oxidation number method. Identify the oxidizing and reducing agents. a. B2O3+MgMgO+Mg3B2 (neutral) b. Cr2O72+Fe2+Cr3++Fe3+ (acidic) c. I2+NO31IO31+NO2 (acidic) d. PbSO4Pb+PbO2+SO42 (acidic) e. Cl1+CrO42ClO1+CrO21 (basic) f. Ni+MnO41NiO+MnO2 (basic) g. I1+Ce4+IO31+Ce3+ (basic)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


