Question: Polyacrylamide is prepared by solution polymerization using isobutyryl peroxide as a free radical initiator. Under reaction conditions, the peroxide half-life is 20 hours. The initiator
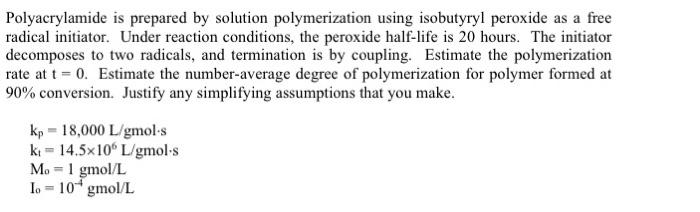
Polyacrylamide is prepared by solution polymerization using isobutyryl peroxide as a free radical initiator. Under reaction conditions, the peroxide half-life is 20 hours. The initiator decomposes to two radicals, and termination is by coupling. Estimate the polymerization rate at t = 0. Estimate the number-average degree of polymerization for polymer formed at 90% conversion. Justify any simplifying assumptions that you make. kp = 18,000 L/gmol's kt - 14.5x100 L/gmol.s M. = 1 gmol/L Io = 10* gmol/L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


