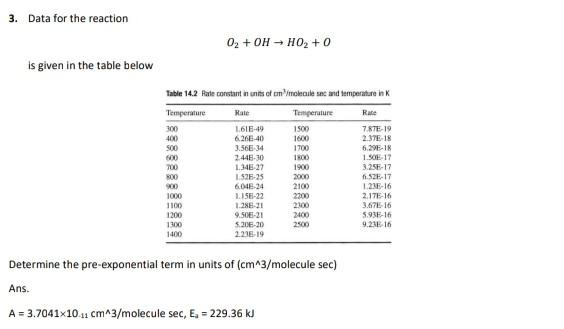
Question: post correct solution with all steps. match your answer before submitting 3. Data for the reaction 02 + OH - HO2 + 0 is given
post correct solution with all steps. match your answer before submitting
3. Data for the reaction 02 + OH - HO2 + 0 is given in the table below Table 14.2 Rate constant in units of molecules and temperature in Rate Temperature 300 400 500 600 700 300 900 1000 1100 1200 L618-49 6.266-40 3.56E 34 2.448-30 1.34 27 152-25 601-24 1.15E-22 1.28E-21 9.50-21 520E-20 2238-19 Temperature 1500 1600 1700 IRO 1900 2000 2100 2200 2800 2400 Rate 7.67E-19 2.376-18 6.295-18 1.50E 17 3.25-17 11.52E-17 1.21E-16 2.17E-16 3.67 16 5.938-16 9.23 16 1300 1400 Determine the pre-exponential term in units of (cm^3/molecule sec) Ans. A = 3.7041x10 11 cm^3/molecule sec, E. = 229.36 kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


