Question: post correct solution with all steps shown. match your answer before submitting 2. Consider the following uni-molecular reaction H,02 + M - OH + OH
post correct solution with all steps shown. match your answer before submitting
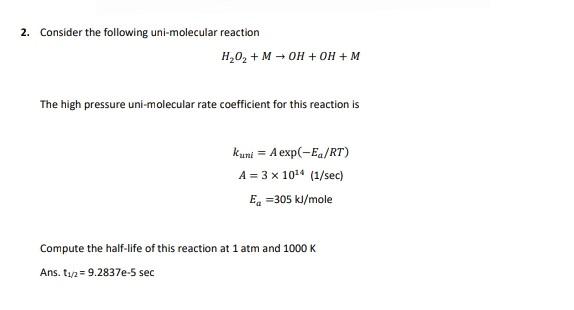
2. Consider the following uni-molecular reaction H,02 + M - OH + OH + M The high pressure uni-molecular rate coefficient for this reaction is kuni = A exp(-E/RT) A = 3 x 1014 (1/sec) Ea=305 kl/mole Compute the half-life of this reaction at 1 atm and 1000K Ans. 112 = 9.2837e-5 sec
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


