Question: POSTLAB Data Table 5. Orders of Reactants in the Rate Law rate = km. [H202] (H+]P Record your data from Activity 2 into the appropriate
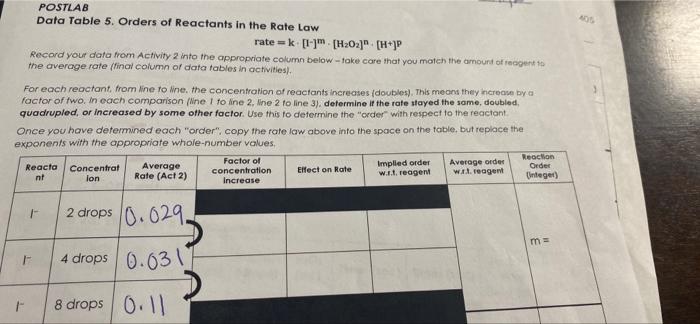
POSTLAB Data Table 5. Orders of Reactants in the Rate Law rate = km. [H202]" (H+]P Record your data from Activity 2 into the appropriate column below take care that you match the amount of reagent the average rate final column of data tables in activities). For each reactant from line to ne, the concentration of reactants increases (doubles). This means they increase by a factor of two. In each comparison (ne I to line 2. Mine 2 to line 3), determine if the rate stayed the same, doubled, quadrupled, or increased by some other factor. Use this to determine the order with respect to the reactant Once you have determined each "order", copy the rate law above into the space on the table, but replace the exponents with the appropriate whole-number values, Factor of Reaction Reacta Concentrat Average Implied order Average order Order Elfect on Rate nt concentration Rate (Act 2) w.1.1. reagent lon W reagent Increase Integer) 2 drops 0.029. ma 4 drops 0.031 8 drops 0.11
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


