Question: Pr.3 Indicate the tendency for corrosion to occur for(12 pints): a) Tin in HCl acid. b) Nickel in silver nitrates. c) Lead in stannous chloride.
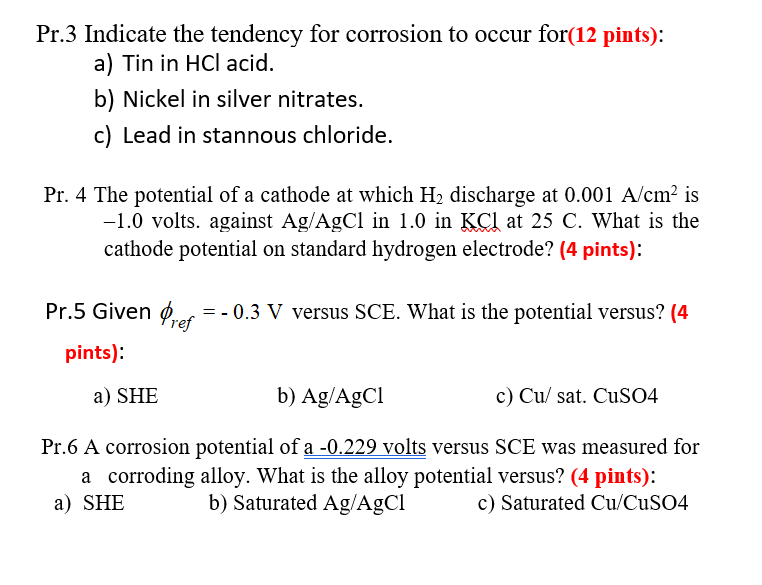
Pr.3 Indicate the tendency for corrosion to occur for(12 pints): a) Tin in HCl acid. b) Nickel in silver nitrates. c) Lead in stannous chloride. Pr. 4 The potential of a cathode at which H2 discharge at 0.001 A/cm is -1.0 volts. against Ag/AgCl in 1.0 in KCl at 25 C. What is the cathode potential on standard hydrogen electrode? (4 pints): Pr.5 Given Prep =- 0.3 V versus SCE. What is the potential versus? (4 pints): a) SHE b) Ag/AgCl c) Cu/ sat. CuSO4 Pr.6 A corrosion potential of a -0.229 volts versus SCE was measured for a corroding alloy. What is the alloy potential versus? (4 pints): a) SHE b) Saturated Ag/AgCl c) Saturated Cu/CuSO4
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


