Question: Pr.9 (15 points) Iron (d=7.8g/cm3) is corroding in a solution saturated with oxygen. The partial pressure of oxygen is 1atm.,bc=0.11V,ic0=107A/cm2,pH=3, ba=+0.08V,ia0=105A2cm2,[Fe2+]=0.7M. Calculate: a) The corrosion
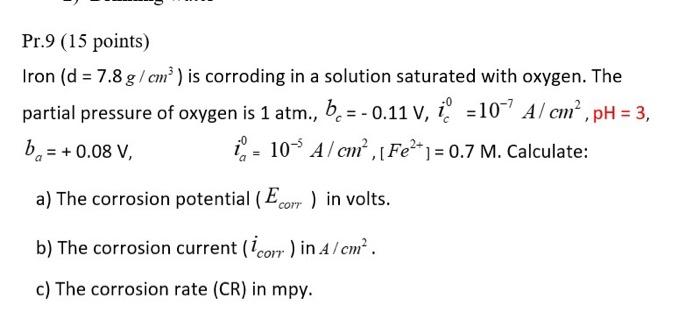
Pr.9 (15 points) Iron (d=7.8g/cm3) is corroding in a solution saturated with oxygen. The partial pressure of oxygen is 1atm.,bc=0.11V,ic0=107A/cm2,pH=3, ba=+0.08V,ia0=105A2cm2,[Fe2+]=0.7M. Calculate: a) The corrosion potential (Ecorr) in volts. b) The corrosion current (icorr) in A/cm2. c) The corrosion rate (CR) in mpy
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


