Question: solve the following question based on given two trials. Molarity of KMnO4 titrant: 1. Calculate the moles of oxalate, C2O42, in each titrated sample of
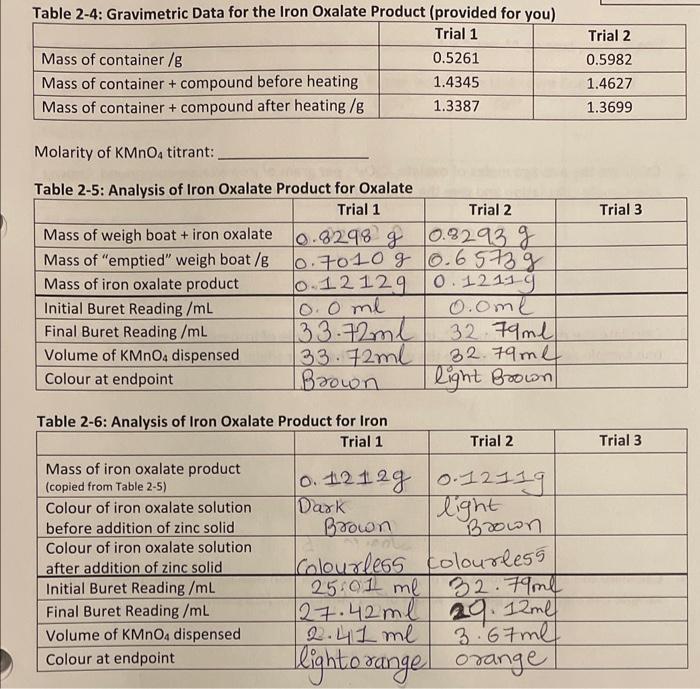


Molarity of KMnO4 titrant: 1. Calculate the moles of oxalate, C2O42, in each titrated sample of iron oxalate compound. Show one sample calculation and give the values of the other trials. [2 points] 2. Calculate the average moles of oxalate, C2O42, per gram of iron oxalate compound. Show one sample calculation and give the values of the other trials. [1.5 points] 3. Calculate the moles of iron, Fen+, in each titrated sample of iron oxalate compound. Show one sample calculation and give the values of the other trials. [ 2 points] 4. Calculate the average moles of iron, Fen, per gram of iron oxalate compound. Show one sample calculation and give the values of the other trials. [1.5 points] 5. Using the gravimetric analysis data provided in Table 2-4, calculate the average moles of water per gram of iron oxalate compound. Show one sample calculation and give the values of the other trials. [ 3 points] 6. Complete the following table. Show sample calculations for * and ** on next page. [3 pts] Sample calculations for * and ** items from Table 2-7: Molarity of KMnO4 titrant: 1. Calculate the moles of oxalate, C2O42, in each titrated sample of iron oxalate compound. Show one sample calculation and give the values of the other trials. [2 points] 2. Calculate the average moles of oxalate, C2O42, per gram of iron oxalate compound. Show one sample calculation and give the values of the other trials. [1.5 points] 3. Calculate the moles of iron, Fen+, in each titrated sample of iron oxalate compound. Show one sample calculation and give the values of the other trials. [ 2 points] 4. Calculate the average moles of iron, Fen, per gram of iron oxalate compound. Show one sample calculation and give the values of the other trials. [1.5 points] 5. Using the gravimetric analysis data provided in Table 2-4, calculate the average moles of water per gram of iron oxalate compound. Show one sample calculation and give the values of the other trials. [ 3 points] 6. Complete the following table. Show sample calculations for * and ** on next page. [3 pts] Sample calculations for * and ** items from Table 2-7
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


